Samarium (III) Selective Membrane Sensor Based on Tin (IV) Boratophosphate
Abstract
:Introduction
Experimental
Reagents
Preparation of exchanger
Preparation of membranes
Preparation of epoxy resin-based membranes
Preparation of PVC membranes
Preparation of PS membranes
EMF measurements
Results and Discussions
Optimization of membrane composition
Calibration curve and statistical data
Effect of internal solution concentration
Effect of pH
Selectivity coefficients and analytical properties of Sm (III) selective electrode
Effect of partially non-aqueous medium on the working of Sm (III) electrode
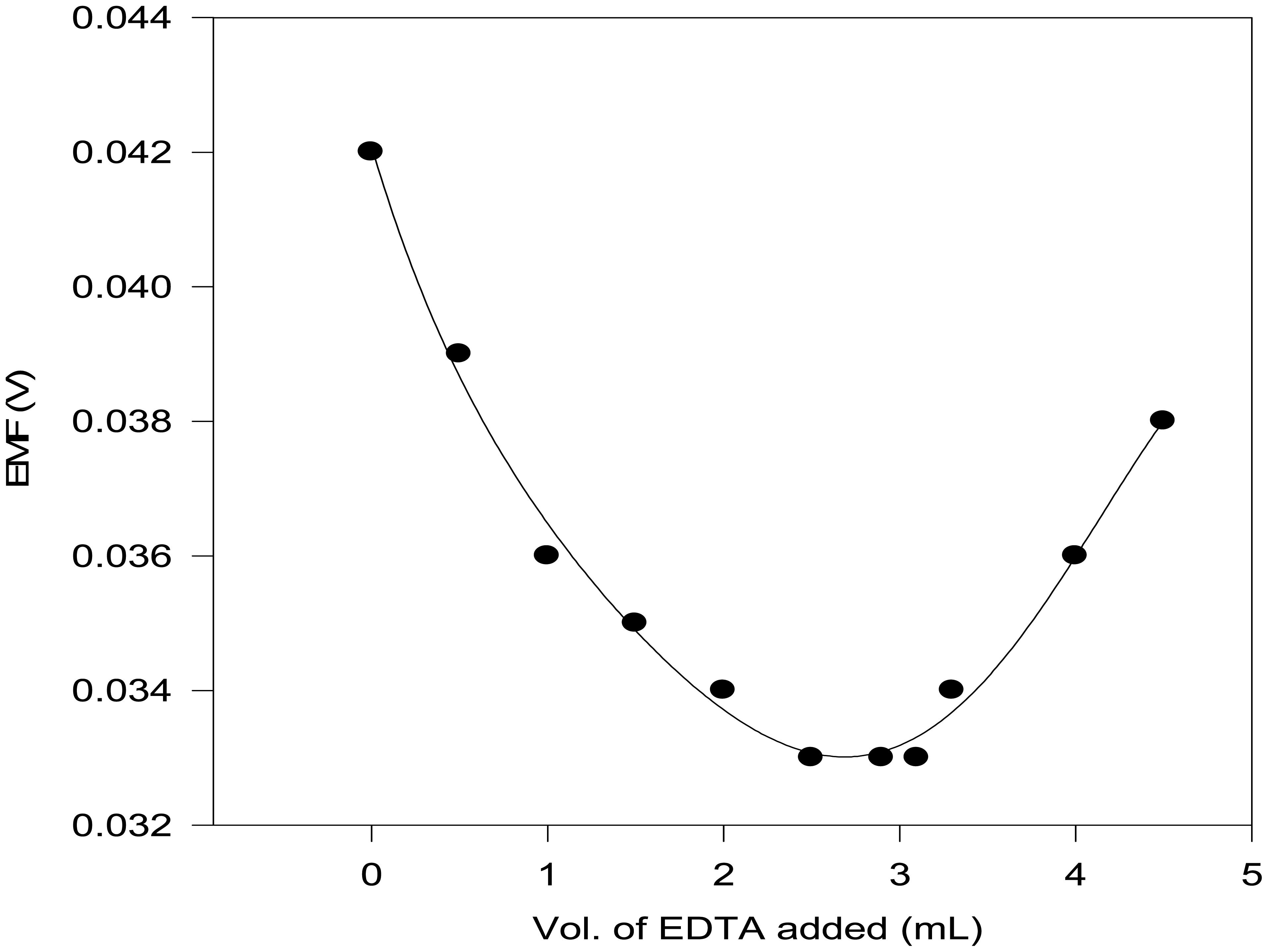
Potentiometric titration
Acknowledgments
References
- Hopkins, B.S. Chemistry of the Rarer Elements; D.C. Heath and Company: Boston, 1923; p. 93. [Google Scholar]
- Ganjali, M.R.; Pourjavid, M.R.; Rezapour, M.; Haghgoo, S. Novel samarium (III) selective membrane sensor based on glipizid. Sensors and Actuators B 2003, 89, 21. [Google Scholar]
- Kirk, O.R.; Othmer, D.F. Encyclopedia of Chemical Technology; Wiley: New York, 1982; Volume 19, p. 851. [Google Scholar]
- Si, Z.; Wang, L.; Hu, J.; Jiang, W. Enhanced luminescence of terbium-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid with lanthanum and its applications. J. Microchem. 2001, 70, 19. [Google Scholar]
- Hrdlicka, A.; Havei, J.; Moreno, C.; Valiente, M. Micellar-enhanced highly sensitive reaction of rare earths with xylenol orange and surfactants. Study of reaction conditions and optimization of spectrophotometric method. Anal. Sci. 1991, 7, 925. [Google Scholar]
- Mazzucotelli, A.; Depaz, F.; Magi, E.; Frache, B. Interferences of major elements in the determinations of rare earth elements by inductively coupled plasma atomic emission spectroscopy. Anal. Sci. 1992, 8, 189. [Google Scholar]
- Masuda, A.; Nakamura, N.; Tanaka, T. Fines structures of mutually normalized rare earth patterns of chondrites. Geochim. Cosmochim. Acta 1973, 37, 239. [Google Scholar]
- Marsh, S.F. Separation of lanthanide fission products from nuclear fuels by extraction chromatography and cation exchange for isotope dilution mass spectrometric analysis. Anal. Chem. 1967, 39, 641. [Google Scholar]
- Chowdhury, D.A.; Ogata, T.; Kamata, S. Samarium (III)-selective electrode using neutral bis(thialkylxanthato)alkanes. Anal. Chem. 1996, 68, 366. [Google Scholar]
- Ogata, T.; Chowdhury, D.A.; Kamata, S.; Usui, Y.; Ohashi, K. Neutral 1,4-bis(3-thiapentylxanthato)butane as sensing material for samarium(III) ion. Chem. Lett. 1995, 24, 1041. [Google Scholar]
- Jammal, A.E.; Bouklouze, A.A.; Patriarche, G.J.; Christian, G.D. Use of ethylene-vinyl-acetate as new membrane matrix for calcium ion-selective electrode preparation. Talanta 1991, 38, 929. [Google Scholar]
- Arnold, M.A.; Solsky, R.L. Ion-selective electrode. Anal. Chem. 1986, 58, 84R. [Google Scholar]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion-selective electrodes (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 2527. [Google Scholar]
- Kraus, K.A.; Phillips, H.O. Adsorption on inorganic materials - I. Cation exchange properties of zirconium phosphate. J. Am. Chem. Soc. 1956, 78, 694. [Google Scholar]
- Kraus, K.A.; Phillips, H.O.; Carlson, T.A.; Johnson, J.S. Basic chemistry in nuclear energy. 2nd UN Conf. Peaceful Uses At. Energy, Geneva 1958, 28, 3. [Google Scholar]
- Amphlett, C.B. 2nd UN Conf. Peaceful Uses At Energy, Geneva 1958, 28, 17.
- Amphlett, C.B. Inorganic Ion Exchangers; Elsevier: Amsterdam, 1964. [Google Scholar]
- Bakker, E. Selectivity of liquid membrane ion selective electrodes. Electroanalysis 1997, 9, 7. [Google Scholar]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: recommended methods for reporting KPot.A,B values (technical report). Pure Appl. Chem. 1995, 67, 507. [Google Scholar]
- Jain, A.K.; Singh, R.P. Srivastava, S.K.; Agrawal, S. Studies with inorganic ion-exchange membranes. Talanta 1978, 25, 157. [Google Scholar]
- Malik, W.U.; Srivastava, S.K.; Bansal, A. Potentiometric estimation of molybdate ion with a solid membrane electrode. Anal. Chem. 1982, 54, 1399. [Google Scholar]
- Moody, G.J.; Thomas, J.D.R. Development and publication of work with selective ion-sensitive electrodes. Talanta 1972, 19, 623. [Google Scholar]
- Harsanyi, E.G.; Toth, K.; Polos, L.; Pungor, E. Adsorption phenomenon of silver-iodide based ionselective electrode. Anal. Chem. 1982, 54, 1094. [Google Scholar]
- Harsanyi, E.G.; Toth, K.; Pungor, E. The behavior of the silver sulphide precipitate based ion-selective electrode in the low concentration range. Anal. Chim. Acta 1984, 161, 333. [Google Scholar]
- .




| Electrode No. | SnBP(%) | Binder | Medium | Slope (mV/decade) | Measuring Range (M) | Response Time (s) |
|---|---|---|---|---|---|---|
| 1 | 30 | PVC | THF | 40 | 2.0×10-5-10-1 | 20 |
| 2 | 40 | PVC | THF | 42 | 2.0×10-5-10-1 | 20 |
| 3 | 10 | Araldite | - | 40 | 1.0×10-5-10-1 | 15 |
| 4 | 20 | Araldite | - | 40 | 2.5×10-5-10-1 | 15 |
| 5 | 30 | Araldite | - | 42 | 1.0×10-5-10-1 | 15 |
| 6 | 40 | Araldite | - | 40 | 1.0×10-5-10-1 | 10 |
| 7 | 40 | Polystyrene | - | 42 | 2.0×10-5-10-1 | 15 |
| Interfering ion (B) | Selectivity Coefficient values (KpotA,B) | |
|---|---|---|
| Interfering ion concentration | ||
| 10-3 M | 10-4 M | |
| La(III) | 0.31 | 0.40 |
| Ce(III) | 0.25 | 0.31 |
| Nd(III) | 0.40 | 0.50 |
| Eu(III) | 0.40 | 0.50 |
| Pr(III) | 0.50 | 0.63 |
| Tb(III) | 0.32 | 0.40 |
| Dy(III) | 0.40 | 0.40 |
| Y(III) | 0.31 | 0.40 |
| Fe(III) | 0.25 | 0.25 |
| Al(III) | 0.10 | 0.31 |
| Ca(II) | 0.50 | 0.50 |
| Na(I) | 0.50 | 0.63 |
| Solvent | Percentage(v/v) | Slope (mV/decade) | Measuring range(M) |
|---|---|---|---|
| Acetone | 5 | 40.0 | 1.0×10-5 to 10-1 |
| 10 | 40.0 | 3.2×10-5 to 10-1 | |
| 15 | 40.0 | 3.2×10-5 to 10-1 | |
| 20 | 40.0 | 1.0×10-5 to 10-1 | |
| Ethanol | 5 | 40.2 | 1.0×10-5 to 10-1 |
| 10 | 40.1 | 2.0×10-5 to 10-1 | |
| 15 | 40.0 | 1.0×10-5 to 10-1 | |
| 20 | 40.2 | 4.3×10-5 to 10-1 | |
| Acetonitrile | 5 | 40.0 | 1.0×10-5 to 10-1 |
| 10 | 40.1 | 1.0×10-5 to 10-1 | |
| 15 | 40.0 | 2.3×10-5 to 10-1 | |
| 20 | 40.0 | 1.0×10-5 to 10-1 |
© 2004 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non-commercial purposes.
Share and Cite
Mittal, S.K.; Sharma, H.K.; Kumar, A.S.K. Samarium (III) Selective Membrane Sensor Based on Tin (IV) Boratophosphate. Sensors 2004, 4, 125-135. https://doi.org/10.3390/s40800125
Mittal SK, Sharma HK, Kumar ASK. Samarium (III) Selective Membrane Sensor Based on Tin (IV) Boratophosphate. Sensors. 2004; 4(8):125-135. https://doi.org/10.3390/s40800125
Chicago/Turabian StyleMittal, Susheel K., Harish Kumar Sharma, and Ashok S. K. Kumar. 2004. "Samarium (III) Selective Membrane Sensor Based on Tin (IV) Boratophosphate" Sensors 4, no. 8: 125-135. https://doi.org/10.3390/s40800125
APA StyleMittal, S. K., Sharma, H. K., & Kumar, A. S. K. (2004). Samarium (III) Selective Membrane Sensor Based on Tin (IV) Boratophosphate. Sensors, 4(8), 125-135. https://doi.org/10.3390/s40800125




