Classification of Mixtures of Odorants from Livestock Buildings by a Sensor Array (an Electronic Tongue)
Abstract
:1. Introduction
2. Experimental
2.1. Sensor array, i.e. the electronic tongue (ET)
2.2. Test mixtures of key odorants
2.3. Experimental design
2.4. Back propagation artificial neural networks
3. Results and discussion
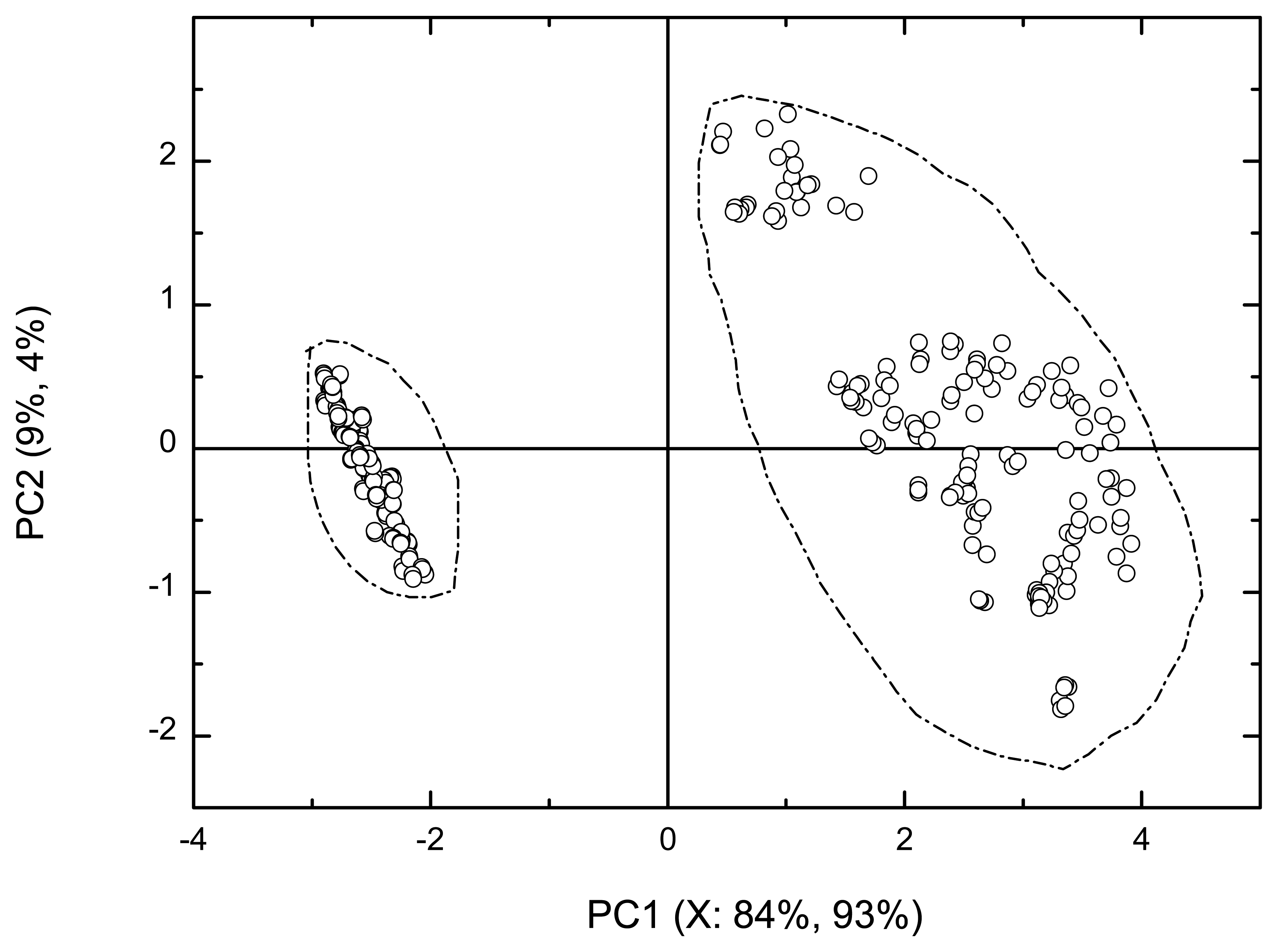
3.1. Classification of test mixtures of key odorants at pH 6
3.2. Classification of test mixtures of key odorants at pH 8
3.3. Classification of test mixtures of key odorants containing ammonium at pH 6 and pH 8
3.4. Classification of test mixtures of key odorants containing p-cresol at pH 6 and pH 8
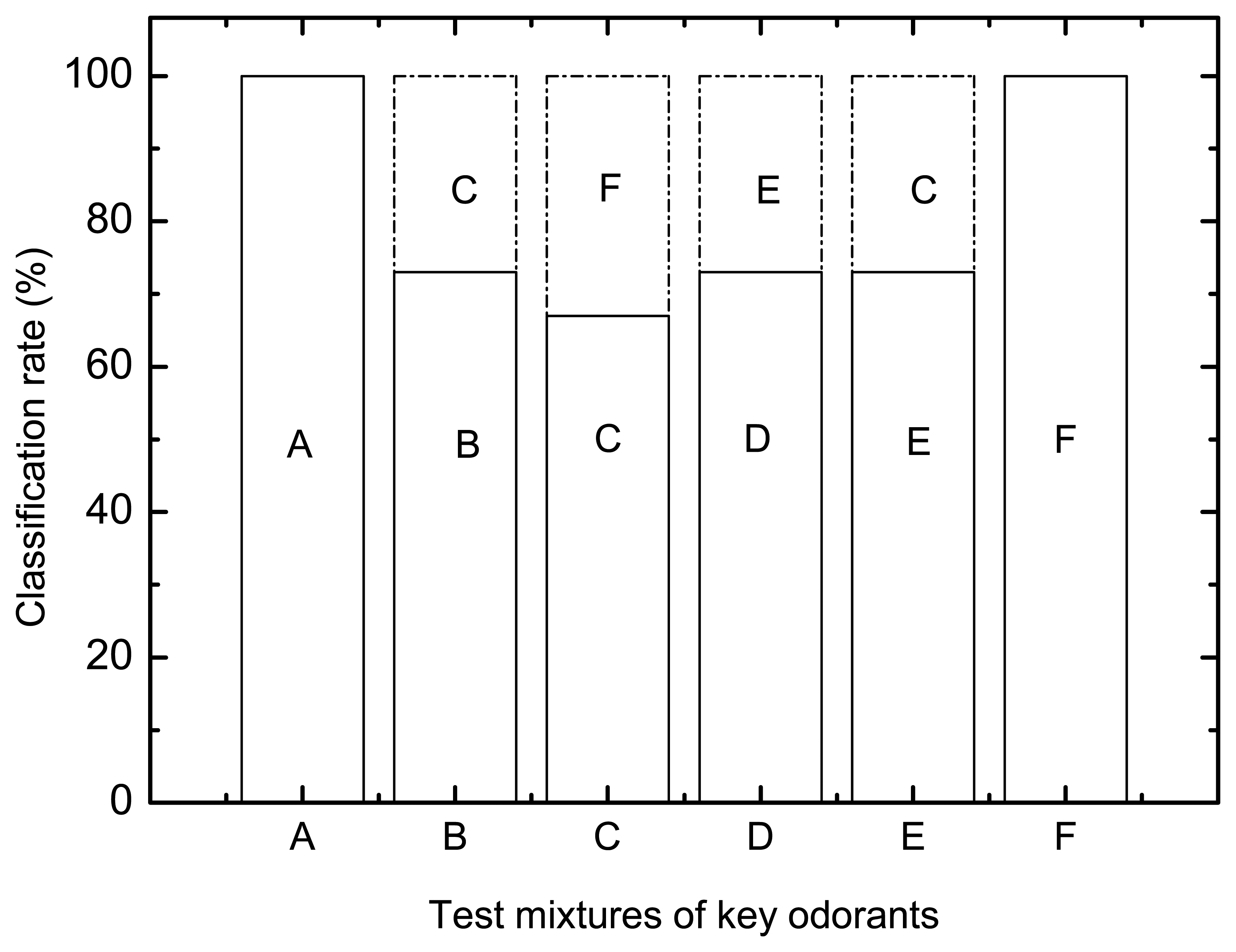
3.5. Classification of test mixtures of key odorants comprising maximum number of combinations of a variety of chemical groups at pH 6
4. Conclusion
Acknowledgments
References
- Schiffman, S. S.; Miller, E. A. S.; Suggs, M. S.; Graham, B. G. The effect of environmental odors emanating from commercial swine operations on the mood of nearby residents. Brain Res. Bull. 1995, 37, 369–375. [Google Scholar]
- Revah, S.; Morgan-Sagastume, J. M. Biotechnology for odor and air pollution; Shareefdeen, Z., Singh, A., Eds.; Springer: New York, 2005; Vol. 1, Chapter 3; p. 29. [Google Scholar]
- Abu-Khalaf, N.; Iversen, J. J. L. Calibration of a sensor array (an electronic tongue) for identification and quantification of odorants from livestock buildings. Sensors 2007, 7, 103–128. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A. Electronic tongues and their analytical application -review. Anal. Bioanal. Chem. 2002, 373, 136–146. [Google Scholar]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Davide, F.; D' Amico, A. Tasting of beverages using an electronic tongue. Sens. Actuator B-Chem. 1997, 44, 291–296. [Google Scholar]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Mazzone, E.; D' Amico, A. Application of electronic tongue for quantitative analysis of mineral water and wine. Electroanalysis 1999, 11, 814–820. [Google Scholar]
- Soderstrom, C.; Rudnitskaya, A.; Legin, A.; Krantz-Rulcker, C. Differentiation of four Aspergillus species and one Zygosaccharomyces with two electronic tongues based on different measurement techniques. J. Biotechnol. 2005, 119, 300–308. [Google Scholar]
- Winquist, F.; Krantz-Rulcker, C.; Lundstrom, I. Electronic tongues. MRS Bull. 2004, 29, 726–731. [Google Scholar]
- Singh, A.; Ward, O. Biotechnology for odor and air pollution; Shareefdeen, Z., Singh, A., Eds.; Springer: New York, 2005; Vol. 1, Chapter 5; p. 101. [Google Scholar]
- McNevin, D.; Barford, J. Biofiltration as an odour abatement strategy. Biochem. Eng. J. 2000, 5, 231–242. [Google Scholar]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar]
- Schiffman, S. S.; Bennett, J. L.; Raymer, J. H. Quantification of odors and odorants from swine operations in North Carolina. Agr. Forest Meteorol. 2001, 108, 123–240. [Google Scholar]
- O'Neil, D. H.; Philips, V. R. A review of the control of odor nuisance from livestock buildings: part 3, properties of the odorous substances which have been identified in livestock wastes or in the air around. J. Agr. Eng. Res. 1992, 53, 23–50. [Google Scholar]
- Datta, I.; Allen, G. Biotechnology for odor and air pollution; Shareefdeen, Z., Singh, A., Eds.; Springer: New York, 2005; Vol. 1, Chapter 6; p. 126. [Google Scholar]
- Arogo, J.; Westerman, P. W.; Heber, A. J. A review of ammonia emissions from confined swine feeding operations. Trans. ASAE 2003, 46, 805–817. [Google Scholar]
- Le, P. D.; Aarnink, A. J. A.; Ogink, N. W. M.; Becker, P. M.; Verstegen, M. W. A. Odour from animal production facilities: its relationship to diet. Nutr. Res. Rev. 2005, 18, 3–30. [Google Scholar]
- Holmberg, M.; Eriksson, M.; Krantz-Rulcker, C.; Artursson, T.; Winquist, F.; Lloyd-Spetz, A.; Lundstrom, I. 2nd workshop of the second network on artificial olfactory sensing (NOSE II). Sens. Actuator B-Chem. 2004, 110, 213–223. [Google Scholar]
- Despagne, F.; Massart, D. L. Neural networks in multivariate calibration. Analyst 1998, 123, 157R–178R. [Google Scholar]
- Maier, H. R.; Dandy, G. C. Empirical comparison of various methods for training feed-forward neural networks for salinity forecasting. Water Resour. Res. 1999, 35, 2591–2596. [Google Scholar]
- NeuralWare. NeuralWorks Predict, The complete solution for neural data modeling, user guide; NeuralWare Inc.: Pittsburgh, 2003. [Google Scholar]
- Basheer, I. A.; Hajmeer, M. Artificial neural networks: fundamentals, computing, design, and application. J. Microbiol. Methods 2000, 43, 3–31. [Google Scholar]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar]
- Copper, J. The use of artificial neural networks for knowledge discovery in bioinformatics. TIBETS 2004, 1, 144–153. [Google Scholar]
- Auger, J.; Arnault, I.; Legin, A.; Rudnitskaya, A.; Seleznev, B.; Sparfel, G.; Dore, C. Comparison of gas chromatography-mass spectrometry and electronic tongue analysis for the classification of onions and shallots. Int. J. Environ. Anal. Chem. 2005, 85, 971–980. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D'Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar]
- Legin, A.; Kirsanov, D.; Rudnitskaya, A.; Iversen, J. J. L.; Seleznev, B.; Esbensen, K. H.; Mortensen, J.; Houmoller, L. P.; Vlasov, Y. Multicomponent analysis of fermentation growth media using the electronic tongue (ET). Talanta 2004, 64, 766–772. [Google Scholar]

- fA: VFAs + phenols
- B: VFAs + skatole
- C: VFAs + ammonium
- D: phenols + skatole
- E: skatole + ammonium
- F: phenols + ammonium
- fA: VFAs + phenols
- B: VFAs + skatole
- C: VFAs + ammonium
- D: phenols + skatole
- E: skatole + ammonium
- F: phenols + ammonium


| Test mixtures of key odorants | Odorants present in test mixture | pH | Interval of concentrations (M) | Number of key odorants in test mixtures | Number of measurements |
|---|---|---|---|---|---|
| Containing ammonium | n-butyrate | 6 | 10-7 - 10-3 | 5 | 150 (50 in triplicates) |
| iso-valerate | 10-7 - 10-4 | ||||
| skatole | 10-8 - 10-6 | ||||
| phenolate | 10-7 - 10-5 | ||||
| ammonium | 10-7 - 10-3 | ||||
| Containing p-cresolate | n-butyrate | 6 | 10-7 - 10-3 | 5 | 150 (50 in triplicates) |
| iso-valerate | 10-7 - 10-4 | ||||
| skatole | 10-8 - 10-6 | ||||
| phenolate | 10-7 - 10-5 | ||||
| p-cresolate | 10-7- 10-5 | ||||
| Containing ammonium | same as above | 8 | same as above | 5 | 150 (50 in triplicates) |
| Containing p-cresolate | same as above | 8 | same as above | 5 | 150 (50 in triplicates) |
| Arbitrary name of test mixtures of key odorants | Groups of key odorants in test mixtures | pH | Key odorants in test mixtures | Interval of concentrations (M) | Numbers of key odorants in test mixtures | Number of measurements |
|---|---|---|---|---|---|---|
| A | VFAs + phenols | 6 | n-butyric acid | 10-6 - 5 × 10-4 | 4 | 45 (15 in triplicates) |
| iso-valeric acid | 5 × 10-7 - 5 × 10-5 | |||||
| phenol | 5 × 10-7 - 10-5 | |||||
| p-cresol | 5 × 10-7 - 10-5 | |||||
| B | VFAs + skatole | 6 | n-butyric acid | 10-6 - 5 × 10-4 | 3 | 45 (15 in triplicates) |
| iso-valeric acid | 5 × 10-7 - 5 ×10-5 | |||||
| skatole | 3 × 10-8 - 5 × 10-7 | |||||
| C | VFAs + ammonium | 6 | n-butyric acid | 10-6 - 5 × 10-4 | 3 | 45 (15 in triplicates) |
| iso-valeric acid | 5 × 10-7 - 5 × 10-5 | |||||
| ammonium | 10-6 - 5 × 10-4 | |||||
| D | phenols + skatole | 6 | phenol | 5 × 10-7 - 10-5 | 3 | 45 (15 in triplicates) |
| p-cresol | 5 × 10-7 - 10-5 | |||||
| skatole | 3 × 10-8 - 5 × 10-7 | |||||
| E | skatole + ammonium | 6 | skatole | 3 × 10-8 - 5 × 10-7 | 2 | 45 (15 in triplicates) |
| ammonium | 10-6 - 5 × 10-4 | |||||
| F | phenols + ammonium | 6 | phenol | 5 × 10-7 - 10-5 | 3 | 45 (15 in triplicates) |
| p-cresol | 5 × 10-7 - 10-5 | |||||
| ammonium | 10-6- 5 × 10-4 |
| Test mixtures of key odorants | Containing ammonium | Containing p-cresolate | Containing ammonium | Containing p-cresolate | ||||
|---|---|---|---|---|---|---|---|---|
| pH | 6 | 6 | 8 | 8 | ||||
| Containing ammonium | 6 | 80% | 20% | |||||
| Containing p-cresolate | 6 | 3% | 97% | |||||
| ACR | 88% | |||||||
| Containing ammonium | 8 | 100% | 0% | |||||
| Containing p-cresolate | 8 | 0% | 100% | |||||
| ACR | 100% | |||||||
| Containing ammonium | 6 | 100% | 0% | |||||
| Containing ammonium | 8 | 0% | 100% | |||||
| ACR | 100% | |||||||
| Containing p-cresolate | 6 | 100% | 0% | |||||
| Containing p-cresolate | 8 | 0% | 100% | |||||
| ACR | 100% | |||||||
| pH | Test mixture of key odorants | Electrode no. | StDevh (mV) | RSDi (%) |
|---|---|---|---|---|
| 6 | Containing ammonium | 1 - 14 | 0 - 11 | 0 - 4.8 |
| 1, 2, 5, 6, 7, 8, 9, 11 | 0 - 6.6 | 0 - 3.4 | ||
| 2, 5, 6, 7, 8, 9 | 0 - 5.6 | 0 - 3.4* | ||
| 6 | Containing p-cresolate | 1 - 14 | 0 - 17.3 | 0 - 15.5 |
| 1, 2, 5, 6, 7, 8, 9, 11 | 0.1 - 6.8 | 0 - 3.5 | ||
| 1, 2, 4, 5, 8 | 0 - 6.8 | 0 - 3.5* | ||
| 8 | Containing ammonium | 1 - 14 | 0 - 2.6 | 0 - 8.4 |
| 1, 2, 5, 6, 7, 8, 9, 11 | 0 - 1.6 | 0 - 0.7 | ||
| 1, 2, 4, 5, 7, 8 | 0 - 1.6 | 0 - 0.7* | ||
| 1, 5, 7, 8 | 0 - 1.6 | 0 - 0.7* | ||
| 8 | Containing p-cresolate | 1 - 14 | 0 - 2.1 | high j |
| 1 - 11, 14 | 0 - 2.1 | 0 - 0.9 | ||
| 1, 2, 5, 6, 7, 8, 9, 11 | 0 - 1.6 | 0 - 0.4 | ||
| 2, 5, 6, 7, 8, 9 | 0 - 1.6 | 0 - 0.4* | ||
| 6 | Test mixtures of key odorants comprising a variety of chemical groups at pH 6 | 1 - 14 | 0 - 3.3 | 0 - 2.3 |
| 1, 2, 5, 6, 7, 8, 9, 11 | 0.1 - 3.0 | 0 - 1.2 | ||
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Abu-Khalaf, N.; Iversen, J.J.L. Classification of Mixtures of Odorants from Livestock Buildings by a Sensor Array (an Electronic Tongue). Sensors 2007, 7, 129-143. https://doi.org/10.3390/s7010129
Abu-Khalaf N, Iversen JJL. Classification of Mixtures of Odorants from Livestock Buildings by a Sensor Array (an Electronic Tongue). Sensors. 2007; 7(1):129-143. https://doi.org/10.3390/s7010129
Chicago/Turabian StyleAbu-Khalaf, Nawaf, and Jens Jørgen Lønsmann Iversen. 2007. "Classification of Mixtures of Odorants from Livestock Buildings by a Sensor Array (an Electronic Tongue)" Sensors 7, no. 1: 129-143. https://doi.org/10.3390/s7010129
APA StyleAbu-Khalaf, N., & Iversen, J. J. L. (2007). Classification of Mixtures of Odorants from Livestock Buildings by a Sensor Array (an Electronic Tongue). Sensors, 7(1), 129-143. https://doi.org/10.3390/s7010129




