Assessment of Diaphragm and External Intercostals Fatigue from Surface EMG using Cervical Magnetic Stimulation
Abstract
:1. Introduction
2. Methods
2.1. Experimental design and Participants
2.2. Inspiratory muscle strength
2.3. Maximal voluntary ventilation
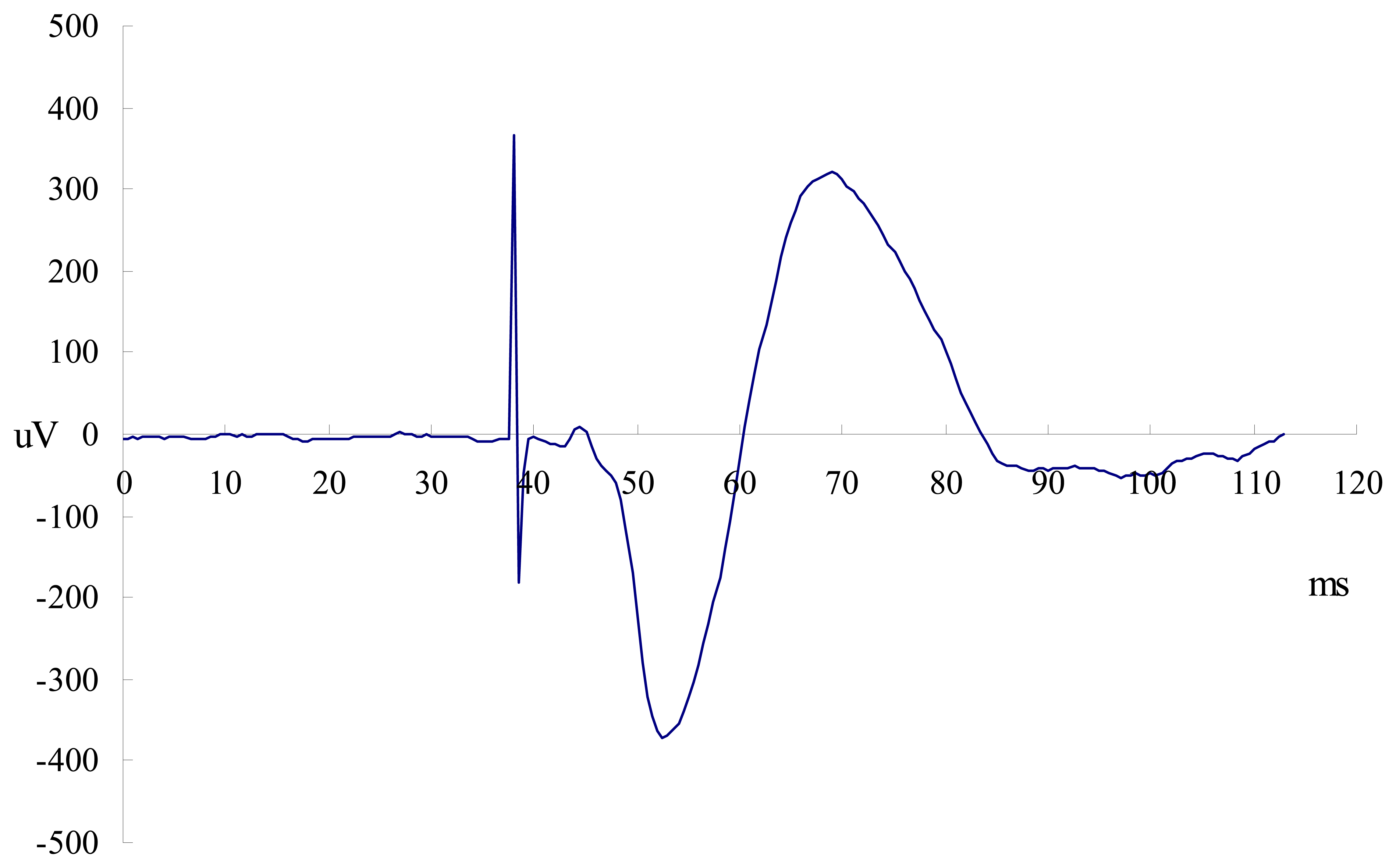
2.4. Cervical magnetic stimulation
2.5. Surface EMG recordings for diaphragm and external intercostals
2.6. Experimental protocol
2.7. EMG data analyses and statistics
3. Results
3.1. Surface EMG parameters in the reliability test
3.2. Maximal inspiratory pressure
3.3. Surface EMG parameters in the MVV trial
4. Discussion
4.1. Reliability of sEMG measures of inspiratory muscles response to CMS
4.2. MVV maneuvers induced diaphragm fatigue but not external intercostals
4.3. sEMG amplitude during voluntary contraction versus evoked CMAP
4.4. Limitations
References
- NHLBI Workshop. Respiratory Muscle Fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am. Rev. Respir. Dis. 1990, 142, 474–480.
- Cohen, C. A.; Zagelbaum, G.; Gross, D.; Roussos, C.; Macklem, P. T. Clinical Manifestations of Inspiratory Muscle Fatigue. Am. J. Med. 1982, 73, 308–316. [Google Scholar]
- Laghi, F.; Topeli, A.; Tobin, M. J. Does Resistive Loading Decrease Diaphragmatic Contractility Before Task Failure? J. Appl. Physiol. 1998, 85, 1103–1112. [Google Scholar]
- Rohrbach, M.; Perret, C.; Kayser, B.; Boutellier, U.; Spengler, C. M. Task Failure from Inspiratory Resistive Loaded Breathing: a Role for Inspiratory Muscle Fatigue? Eur. J. Appl. Physiol. 2003, 90, 405–410. [Google Scholar]
- Mador, M. J.; Dahuja, M. Mechanisms for Diaphragmatic Fatigue Following High-intensity Leg Exercise. Am. J. Respir. Crit. Care. Med. 1996, 154, 1484–1489. [Google Scholar]
- Hamnegård, C.; Wragg, S.; Kryoussis, D.; Mills, G. H.; Polkey, M. I.; Moran, J.; Road, J.; Bake, B.; Green, M.; Moxham, J. Diaphragm Fatigue Following Maximal Ventilation in Man. Eur. Respir. J. 1996, 9, 241–247. [Google Scholar]
- Johnson, B. D.; Babcock, M. A.; Suman, O. E.; Dempsey, J. A. Exercise-induced Diaphragmatic Fatigue in Healthy Humans. J. Physiol. 1993, 460, 385–405. [Google Scholar]
- Mador, M. J.; Magalang, U. J.; Rodis, A.; Kufel, T. J. Diaphragmatic Fatigue After Exercise in Healthy Human Subjects. Am. Rev. Respir. Dis. 1993, 148, 1571–1575. [Google Scholar]
- Verges, S.; Notter, D.; Spengler, C. M. Influence of Diaphragm and Rib Cage Muscle Fatigue on Breathing during Endurance Exercise. Respir. Physiol. Neurobiol. 2006, 154, 431–442. [Google Scholar]
- Perlovitch, R.; Gefen, A.; Elad, D.; Ratnovsky, A.; Kramer, M. R.; Halpern, P. Inspiratory Muscles Experience Fatigue Faster Than the Calf Muscles during Treadmill Marching. Respir. Physiol. Neurobiol. 2007, 156, 61–68. [Google Scholar]
- Mulvey, D. A.; Koulouris, N. G.; Elliott, M. W.; Laroche, C. M.; Moxham, J.; Green, M. Inspiratory Muscle Relaxation Rate After Voluntary Maximal Isocapnic Ventilation in Humans. J. Appl. Physiol. 1991, 70, 2173–2180. [Google Scholar]
- Kyroussis, D.; Mills, G.; Hamnegård, C. H.; Wragg, S.; Road, J.; Green, M.; Moxham, J. Inspiratory Muscle Relaxation Rate Assessed from Sniff Nasal Pressure. Thorax 1994, 49, 1127–1133. [Google Scholar]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care. Med. 2002, 166, 518–628.
- Rafferty, G. F.; Harris, M. l.; Polkey, M. I.; Greenough, A.; Moxham, J. Effect of Hypercapnia on Maximal Voluntary Ventilation and Diaphragm Fatigue in Normal Humans. Am. J. Respir. Care. Med. 1999, 160, 1567–1571. [Google Scholar]
- McKenzie, D. K.; Gandevia, S. C. Phrenic Nerve Conduction Times and Twitch Pressures of the Human Diaphragm. J. Appl. Physiol. 1985, 58, 1496–1504. [Google Scholar]
- Edwards, R. H. T.; Hill, D. K.; Jones, D. A.; Merton, P. A. Fatigue of Long Duration in Human Skeletal Muscle After Exercise. J. Physiol. 1977, 272, 769–778. [Google Scholar]
- Mier, A.; Brophy, C.; Moxham, J.; Green, M. Phrenic Nerve Stimulation in Normal Subjects and in Patients with Diaphragmatic Weakness. Thorax 1987, 42, 885–888. [Google Scholar]
- Mills, G.; Kyroussis, D.; Hamnegård, C. H.; Wragg, S.; Moxham, J.; Green, M. Chest Wall Activation During Cervical Magnetic Phrenic Stimulation (CMPS) does not Produce Inspiratory Pressures. Am. J. Respir. Crit. Care. Med. 1995, 151, A414. [Google Scholar]
- Similowski, T.; Fleury, B.; Launois, S.; Cathala, H. P.; Bouche, P.; Derenne, J. P. Cervical Magnetic Stimulation: a New Painless Method for Bilateral Phrenic Nerve Stimulation in Conscious Humans. J. Appl. Physiol. 1989, 67, 1311–1318. [Google Scholar]
- Similowski, T.; Mehiri, S.; Duguet, A.; Attali, V.; Straus, C.; Derenne, J. P. Comparison of Magnetic and Electrical Phrenic Nerve Stimulation in Assessment of Phrenic Nerve Conduction Time. J. Appl. Physiol. 1997, 82, 1190–1199. [Google Scholar]
- Similowski, T.; Straus, C.; Attali, V.; Duguet, A.; Jourdain, B.; Derenne, J. P. Assessment of the Motor Pathway to the Diaphragm Using Cortical and Cervical Magnetic Stimulation in the Decision-making Process of Phrenic Pacing. Chest 1996, 110, 1551–1557. [Google Scholar]
- Verin, E.; Straus, C.; Demoule, A.; Mialon, P.; Derenne, J.; Similowski, T. Validation of Improved Recording Site to Measure Phrenic Conduction from Surface Electrodes in Humans. J. Appl. Phsyiol. 2002, 92, 967–974. [Google Scholar]
- Demoule, A.; Verin, E.; Locher, C.; Derenne, J. P.; Similowski, T. Validation of Surface Recordings of the Diaphragm Response to Transcranial Magnetic Stimulation in Humans. J. Appl. Physiol. 2003, 94, 453–461. [Google Scholar]
- Black, L.; Hyatt, R. Maximal Respiratory Pressure: Normal Values and Relationship to Age and Sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar]
- Clanton, T. L.; Diaz, P. T. Clinical Assessment of the Respiratory Muscles. Phys. Ther. 1995, 75, 983–995. [Google Scholar]
- Wen, A.; Woo, S.; Knees, T. G. How Many Maneuvers Are Required to Measure Maximal Inspiratory Pressure Accurately? Chest 1997, 111, 802–807. [Google Scholar]
- Luo, Y. M.; Polkey, M. I.; Johnson, L. C.; Lyall, R. A.; Harris, M. L.; Green, M.; Moxham, J. Diaphragm EMG Measured by Cervical Magnetic and Electrical Phrenic Nerve Stimulation. J. Appl. Physiol. 1998, 85, 2089–2099. [Google Scholar]
- Duiverman, M. L.; van Eykern, L. A.; Vennik, P. W.; Koeter, G. H.; Maarsingh, E. J.; Wijkstra, P. J. Reproducibility and Responsiveness of a Noninvasive EMG Technique of the Respiratory Muscles in COPD Patients and in Healthy Subjects. J. Appl. Physiol. 2004, 96, 1723–1729. [Google Scholar]
- Man, W. D.; Luo, Y. M.; Mustfa, N.; Rafferty, G. F.; Glerant, J. C.; Polkey, M. I.; Moxham, J. Postprandial Effects on Twitch Transdiaphragmatic Pressure. Eur. Respir. J. 2002, 20, 577–580. [Google Scholar]
- Sinderby, C.; Friberg, S.; Comtois, N.; Grassino, A. Chest Wall Muscle Cross Talk in Canine Costal Diaphragm Electromyogram. J. Appl. Physiol. 1996, 81, 2312–2327. [Google Scholar]
- Glerant, J. C.; Man, W. D.; Luo, Y. M.; Rafferty, G.; Polkey, M. I.; Moxham, J. Diaphragm Electromyograms Recorded from Multiple Surface Electrodes Following Magnetic Stimulation. Eur. Respir. J. 2006, 27, 334–342. [Google Scholar]
- Maarsingh, E. J.; van Eykern, L. A.; Sprikkelman, A. B.; Hoekstra, M. O.; van Aalderen, W. M. Respiratory Muscle Activity Measured with a Noninvasive EMG Technique: Technical Aspects and Reproducibility. J. Appl. Physiol. 2000, 88, 1955–1961. [Google Scholar]
- Jonville, S.; Jutand, L.; Similowski, T.; Denjean, A.; Delpech, N. Putative Protective Effect of Inspiratory Threshold Loading Against Exercise-induced Supraspinal Diaphragm Fatigue. J. Appl. Physiol. 2005, 98, 991–998. [Google Scholar]
- Ozkaplan, A.; Rhodes, E. C.; Sheel, A. W.; Taunton, J. E. A Comparison of Inspiratory Muscle Fatigue Following Maximal Exercise in Moderately Trained Males and Females. Eur. J. Appl. Physiol. 2005, 95, 52–56. [Google Scholar]
- Volianitis, S.; McConnell, A. K.; Jones, D. A. Assessment of Maximum Inspiratory Pressure. Prior Submaximal Respiratory Muscle Activity (‘Warm-up’) Enhances Maximum Inspiratory Activity and Attenuates the Learning Effect of Repeated Measurement. Respiration 2001, 68, 22–27. [Google Scholar]
- De Luca, C. The Use of Surface Electromyograhy in Biomechanics. J. Appl. Biomech. 1997, 13, 153–163. [Google Scholar]
- Roussos, C.; Fixley, M.; Gross, D.; Macklem, P. T. Fatigue of Inspiratory Muscles and Their Synergic Behavior. J. Appl. Physiol. 1979, 46, 897–904. [Google Scholar]
- Hunter, S. K.; Enoka, R. M. Changes in Muscle Activation can Prolong the Endurance Time of a Submaximal Isometric Contraction in Humans. J. Appl. Physiol. 2003, 94, 108–118. [Google Scholar]
- De Luca, C. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 2000, 13, 135–163. [Google Scholar]
- Bigland-Ritchie, B.; Donovan, E. F.; Roussos, C. S. Conduction Velocity and EMG Power Spectrum Changes in Fatigue of Sustained Maximal Efforts. J. Appl. Physiol. 1981, 51, 1300–1305. [Google Scholar]
- Bilodeau, M.; Schindler-Ivens, S.; Williams, D. M.; Chandran, R.; Sharma, S. S. EMG Frequency Content Changes with Increasing Force and during Fatigue in the Quadriceps Femoris Muscle of Men and Women. J. Electromyogr. Kinesiol. 2003, 13, 83–92. [Google Scholar]
- De Luca, C. The Use of the Surface EMG Signal for Performance Evaluation of Back Muscle. Muscle. Nerve. 1993, 16, 210–216. [Google Scholar]
- Garland, S. Role of Small Diameter Afferents in Reflex Inhibition during Human Muscle Fatigue. J. Physiol. 1991, 435, 547–558. [Google Scholar]
- Juel, C. The Effect of p2-adrenoceptor Activation on Ion-shifts and Fatigue in Mouse Soleus Muscle Stimulated in Vitro. Acta. Physiol. Scand. 1988, 134, 209–216. [Google Scholar]
- Arendt-Nielsen, L.; Zwarts, M. Measurement of Muscle Fiber Conduction Velocity in Humans: Techniques and Applications. J. Clin. Neurophysiol. 1989, 6, 173–190. [Google Scholar]
- Bigland-Ritchie, B.; Thomas, C.K.; Rice, C. L.; Howarth, J. V.; Woods, J. J. Muscle Temperature, Contractile Speed, and Motoneuron Firing Rates during Human Voluntary Contractions. J. Appl. Physiol. 1992, 73, 2457–2461. [Google Scholar]
- Viitasalo, J.T.; Komi, P. V. Interrelationships of EMG Signal Characteristics at Different Levels of Muscle Tension and during Fatigue. Electromyogr. Clin. Neurophysiol. 1978, 18, 167–178. [Google Scholar]
- Bilodeau, M.; Arsenault, A.; Gravel, D.; Bourbonnais, D. EMG power spectra of elbow extensor during ramp and step isometric contraction. Eur. J. Appl. Phsiol. 1991, 63, 24–28. [Google Scholar]
- Bilodeau, M.; Schindler-Ivens, S.; Williams, D. M.; Chandran, R.; Sharma, S. S. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. J. Electromyogr. Kinesiol. 2003, 13, 83–92. [Google Scholar]
- Grassino, A.; Clanton, T. Respiratory Muscle Fatigue. Sem. Resp. Med. 1991, 12, 305–321. [Google Scholar]
- Smith-Blair, N. Mechanisms of Diaphragm Fatigue. AACN. Clinical. Issues. 2002, 13, 307–319. [Google Scholar]

| Pretest | Posttest | ICC* | P value# | |
|---|---|---|---|---|
| PImax (cmH2O) | 59.6±13.5 | 59.0±13.9 | 0.99 | 0.394 |
| Diaphragm | ||||
| RMS (μV) | 60.2±12.9 | 58.8±12.7 | 0.99 | 0.054 |
| Median frequency (Hz) | 104.0±17.0 | 103.2±17.5 | 0.98 | 0.635 |
| CMAP latency (ms) | 5.84±0.44 | 5.75±0.42 | 0.97 | 0.144 |
| CMAP amplitude (μV) | 643.8±315.3 | 672.6±355.4 | 0.99 | 0.064 |
| External intercostals | ||||
| RMS (μV) | 84.1±33.5 | 84.4±34.9 | 0.99 | 0.778 |
| Median frequency (Hz) | 100.1±9.8 | 100.6±11.4 | 0.99 | 0.558 |
| CMAP latency (ms) | 3.38±0.24 | 3.35±0.27 | 0.95 | 0.333 |
| CMAP amplitude (μV) | 874.9±291.2 | 880.3±294.0 | 0.99 | 0.428 |
| Pretest | Posttest | Recovery | p-value | |
|---|---|---|---|---|
| PImax (cmH2O) | 62.2±14.0 | 49.0±16.5* | 56.4±14.1*# | 0.000 |
| Diaphragm | ||||
| RMS (μV) | 58.5±13.2 | 47.9±12.1* | 51.5±15.8* | 0.000 |
| Median frequency (Hz) | 101.8±16.2 | 89.7±14.4* | 91.7±16.7* | 0.000 |
| CMAP latency (ms) | 5.80±0.39 | 5.74±0.40 | 5.80±0.40 | 0.928 |
| CMAP amplitude (μV) | 650.2±314.2 | 619.2±263.5 | 673.0±311.3 | 0.238 |
| External intercostals | ||||
| RMS (μV) | 88.9±44.5 | 80.7±35.5 | 90.5±47.9 | 0.206 |
| Median frequency (Hz) | 100.9±12.9 | 94.3±10.5* | 97.3±13.3 | 0.013 |
| CMAP latency (ms) | 3.42±0.29 | 3.38±0.21 | 3.38±0.23 | 0.408 |
| CMAP amplitude (μV) | 879.6±273.8 | 850.8±262.0 | 879.3±265.0 | 0.057 |
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Chien, M.-Y.; Wu, Y.-T.; Chang, Y.-J. Assessment of Diaphragm and External Intercostals Fatigue from Surface EMG using Cervical Magnetic Stimulation. Sensors 2008, 8, 2174-2187. https://doi.org/10.3390/s8042174
Chien M-Y, Wu Y-T, Chang Y-J. Assessment of Diaphragm and External Intercostals Fatigue from Surface EMG using Cervical Magnetic Stimulation. Sensors. 2008; 8(4):2174-2187. https://doi.org/10.3390/s8042174
Chicago/Turabian StyleChien, Meng-Yueh, Ying-Tai Wu, and Ya-Ju Chang. 2008. "Assessment of Diaphragm and External Intercostals Fatigue from Surface EMG using Cervical Magnetic Stimulation" Sensors 8, no. 4: 2174-2187. https://doi.org/10.3390/s8042174
APA StyleChien, M.-Y., Wu, Y.-T., & Chang, Y.-J. (2008). Assessment of Diaphragm and External Intercostals Fatigue from Surface EMG using Cervical Magnetic Stimulation. Sensors, 8(4), 2174-2187. https://doi.org/10.3390/s8042174




