Electrochemical Assay of Human Islet Amyloid Polypeptide and Its Aggregation
Abstract
:1. Introduction
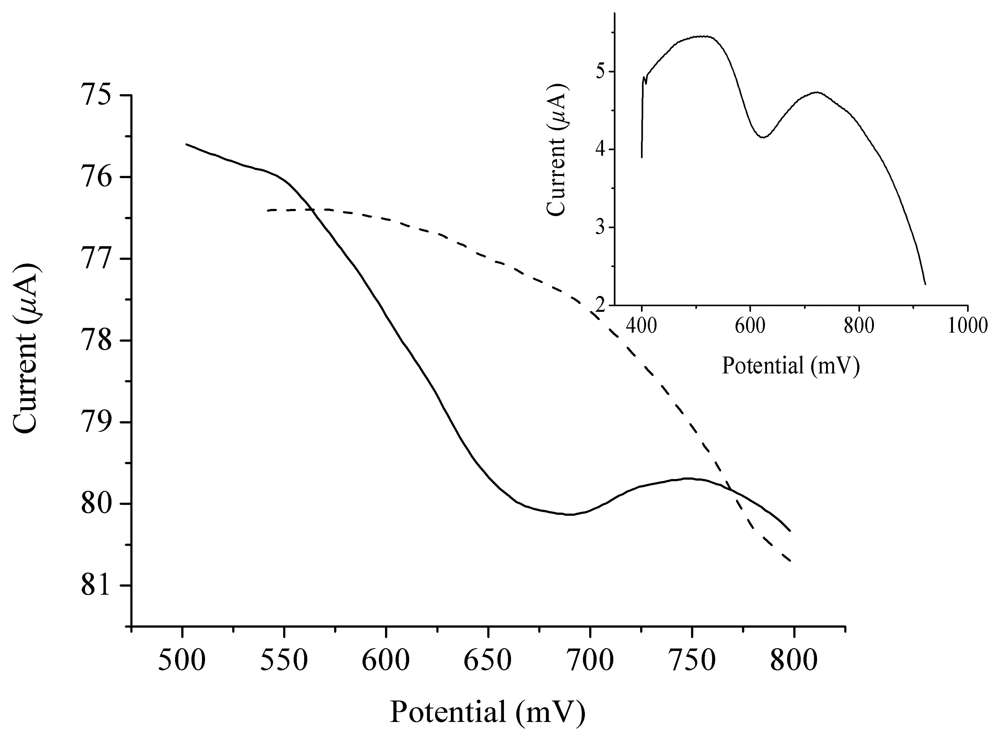
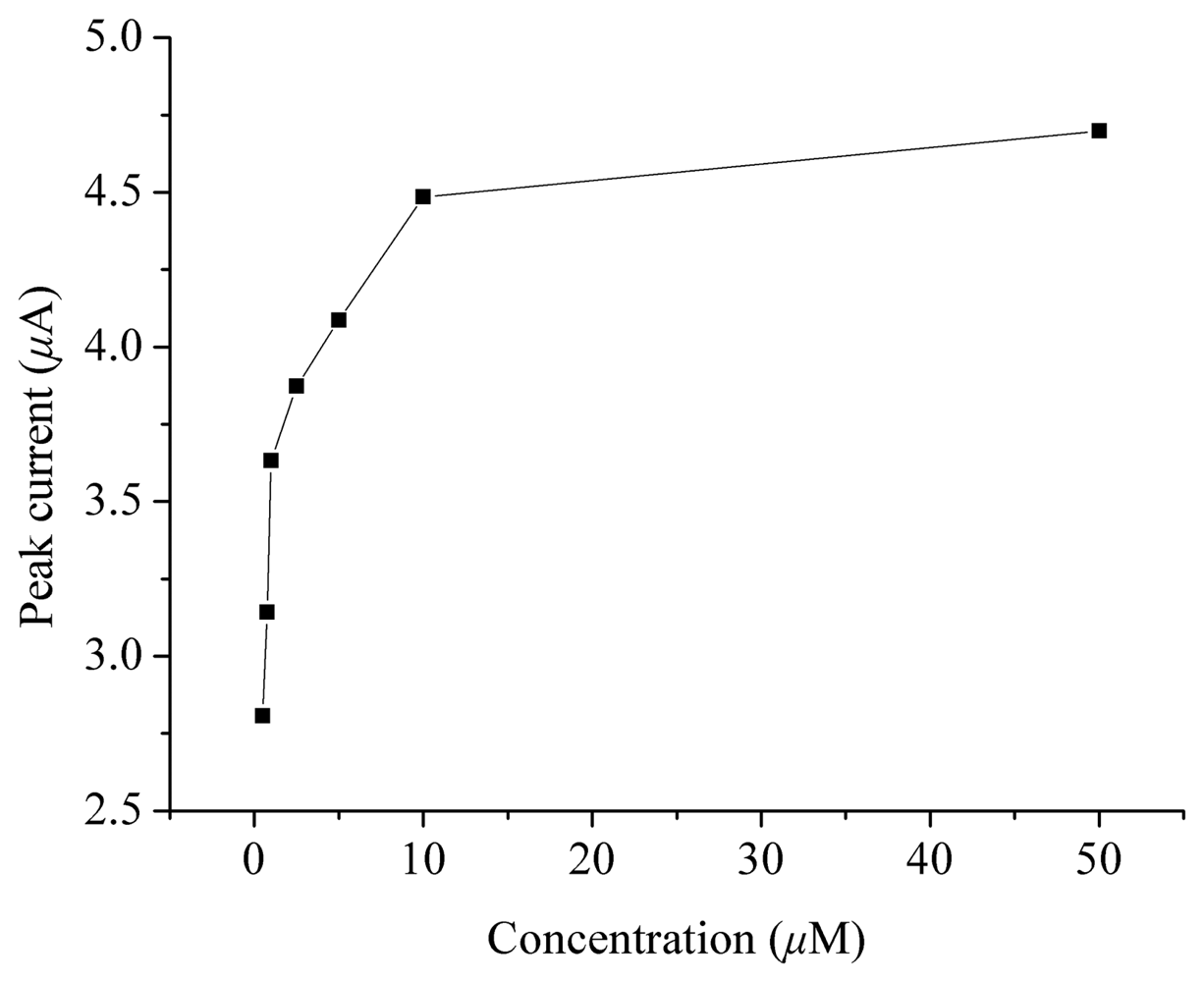
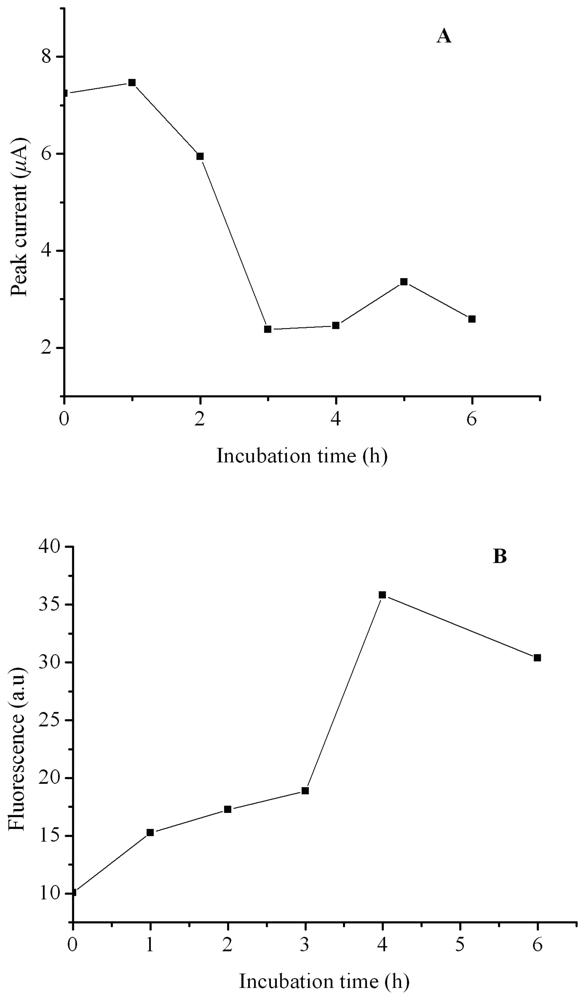
2. Results and Discussion
3. Experimental Section
3.1 Chemicals
3.2 Apparatus
3.3 Preparation of hIAPP solution
3.4 Electrochemical measurement
3.5 Fluorescence study
4. Conclusions
Acknowledgments
References
- Selkoe, D.J. Folding proteins in fatal ways. Nature 2003, 246, 900–904. [Google Scholar]
- Glenner, G.G. Amyloid deposits and amyloidosis: the β-fibrilloses (first of two parts). N. Engl. J. Med. 1980, 302, 1283–1292. [Google Scholar]
- Glenner, G.G. Amyloid deposits and amyloidosis: the β-fibrilloses (second of two parts). N. Engl. J. Med. 1980, 302, 1333–1343. [Google Scholar]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar]
- Cooper, G.; Willis, A.C.; Clark, A.; Turner, R.C.; Sim, R.B.; Reid, K.B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA 1987, 84, 8628–8632. [Google Scholar]
- Westermark, P.; Wernstedt, C.; Wilander, E.; Sletten, K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Commun. 1986, 140, 827–831. [Google Scholar]
- Westermark, P.; Wernstedt, C.; Wilander, E.; Hayden, D.; O'brien, T.; Johnson, K. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from neuropeptide-like protein also present in normal islets. Proc. Natl. Acad. Sci. USA 1987, 84, 3881–3885. [Google Scholar]
- Wimalawansa, S.J. Amylin, caleitonin, caleitonin gene-related peptide, and adrenomdullin: a peptide superfamily. Crit. Rev. Neurobiol. 1997, 11, 167–239. [Google Scholar]
- Schmitz, O.; Brock, B.; Rungby, J. Amylin Agonists: A Novel Approach in the Treatment of Diabetes. Diabetes 2004, 53, S233–S238. [Google Scholar]
- Kapurniotu, A. Amyloidogenicity and cytotoxicity of islet amyloid polypeptide. Biopolymers 2001, 60, 438–459. [Google Scholar]
- Kayed, R.; Bernhagen, J.; Greenfield, N.; Sweimeh, K.; Brunner, H.; Voelter, W.; Kapurniotu, A. Conformational Transitions of Islet Amyloid Polypeptide (IAPP) in Amyloid Formation in Vitro. J. Mol. Biol. 1999, 287, 781–796. [Google Scholar]
- Padrick, S.B.; Miranker, A.D. Islet Amyloid: Phase Partitioning and Secondary Nucleation Are Central to the Mechanism of Fibrillogenesis. Biochemistry 2002, 41, 4694–4703. [Google Scholar]
- Hull, R.L.; Westermark, G.T.; Westermark, P.; Kahn, S.E. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 3629–3643. [Google Scholar]
- Lorenzo, A.; Razzboni, B.; Weir, G.C.; Yankner, B.A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 1994, 368, 756–760. [Google Scholar]
- Anguiano, M.; Nowak, R.; Lansbury, P. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry 2002, 41, 11338–11343. [Google Scholar]
- Porat, Y.; Kolusheva, S.; Jelinek, R.; Gazit, E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry 2003, 42, 10971–10977. [Google Scholar]
- Abedini, A.; Raleigh, D.P. The Role of His-18 in Amyloid Formation by Human Islet Amyloid Polypeptide. Biochemistry 2005, 44, 16284–16291. [Google Scholar]
- Makin, O.S.; Serpell, L.C. Structural Characterisation of Islet Amyloid Polypeptide Fibrils. J. Mol. Biol. 2004, 335, 1279–1288. [Google Scholar]
- Higham, C.E.; Jaikaran, E.; Fraser, P.E.; Gross, M.; Clark, A. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. FEBS. Lett. 2000, 470, 55–60. [Google Scholar]
- Jaikaran, E.; Higham, C.E.; Serpell, L.C.; Zurdo, J.; Gross, M.; Clark, A.; Fraser, P.E. Identification of a novel human islet amyloid polypeptide β-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 2001, 308, 515–525. [Google Scholar]
- Goldsbury, C.; Goldie, K.; Pellaud, J.; Seelig, J.; Frey, P.; Müller, S.A.; Kistler, J.; Cooper, G.; Aebi, U. Amyloid fibril formation from full-length and fragments of amylin. J. Struct. Biol. 2000, 130, 352–362. [Google Scholar]
- Tatarek-Nossol, M.; Yan, L.; Schmauder, A.; Tenidis, K.; Westermark, G.; Kapurniotu, A. Inhibition of hIAPP amyloid-fibril formation and apoptotic cell death by a designed hIAPP amyloid-core-containing hexapeptide. Chem. Biol. 2005, 12, 797–809. [Google Scholar]
- Scrocchi, L.A.; Chen; Waschuk, S.; Wang, F.; Cheung, S.; Darabie, A.A.; McLaurin, J.; Fraser, P.E. Design of peptide-based inhibitors of human islet amyloid polypeptide fibrillogenesis. J. Mol. Biol. 2002, 318, 697–706. [Google Scholar]
- Yan, L.; Tatarek-Nossol, M.; Velkova, A.; Kazantzis, A.; Kapurniotu, A. Design of a mimic of nonamyloidogenic and bioactive human islet amyloid polypeptide (IAPP) as nanomolar affinity inhibitor of IAPP cytotoxic fibrillogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 2046–2051. [Google Scholar]
- Kapurniotu, A.; Schmauder, A.; Tenidis, K. Structure-based design and study of non-amyloidogenic, double N-methylated IAPP amyloid core sequences as inhibitors of IAPP amyloid formation and cytotoxicity. J. Mol. Biol. 2002, 315, 339–350. [Google Scholar]
- Guo, L.w.; Qu, N. Chemical-induced unfolding of cofactor-free protein monitored by electrochemistry. Anal. Chem. 2006, 78, 6275–6278. [Google Scholar]
- Vestergaard, M.; Kerman, K.; Saito, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. A rapid label-free electrochemical detection and kinetic study of Alzheimer's amyloid beta aggregation. J. Am. Chem. Soc. 2005, 127, 11892–11893. [Google Scholar]
- Vestergaard, M.; Kerman, K.; Tamiya, E. An overview of label-free electrochemical protein sensors. Sensors 2007, 7, 3442–3458. [Google Scholar]
- Zhang, W.; Fan, C.; Sun, Y.; Li, G. An electrochemical investigation of ligand-binding abilities of film-entrapped myoglobin. Biochim. Biophys. Acta. Gen. Sub. 2003, 1623, 29–32. [Google Scholar]
- Huang, Y.; Liu, L.; Shi, C.; Huang, J.; Li, G. Electrochemical analysis of the effect of Ca2+ on cardiolipin–cytochrome c interaction. Biochim. Biophys. Acta. Gen. Sub. 2006, 1760, 1827–1830. [Google Scholar]
- Xiao, H.; Wang, J.; Chen, G.; Li, G. Electrochemical evaluation of self-disassociation of PKA upon activation by cAMP. Langmuir 2007, 23, 3506–3508. [Google Scholar]
- Xiao, H.; Zhou, H.; Chen, G.; Liu, S.; Li, G. Interaction between inducible nitric oxide synthase and calmodulin in Ca2+-free and -bound forms. J. Proteome Res. 2007, 6, 1426–1429. [Google Scholar]
- Anguiano, M.; Nowak, R.; Lansbury, P.T.J. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry 2002, 41, 11338–11343. [Google Scholar]
- Green, J.D.; Goldsbury, C.; Kistler, J.; Cooper, G.J.; Aebi, U. Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J. Biol. Chem. 2004, 279, 12206–12212. [Google Scholar]

© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, N.; Chen, Z.; Zhang, D.; Li, G. Electrochemical Assay of Human Islet Amyloid Polypeptide and Its Aggregation. Sensors 2008, 8, 5987-5995. https://doi.org/10.3390/s8095987
Zhou N, Chen Z, Zhang D, Li G. Electrochemical Assay of Human Islet Amyloid Polypeptide and Its Aggregation. Sensors. 2008; 8(9):5987-5995. https://doi.org/10.3390/s8095987
Chicago/Turabian StyleZhou, Nandi, Zhenyu Chen, Dongmei Zhang, and Genxi Li. 2008. "Electrochemical Assay of Human Islet Amyloid Polypeptide and Its Aggregation" Sensors 8, no. 9: 5987-5995. https://doi.org/10.3390/s8095987
APA StyleZhou, N., Chen, Z., Zhang, D., & Li, G. (2008). Electrochemical Assay of Human Islet Amyloid Polypeptide and Its Aggregation. Sensors, 8(9), 5987-5995. https://doi.org/10.3390/s8095987



