Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Nylon-6 nanofibers
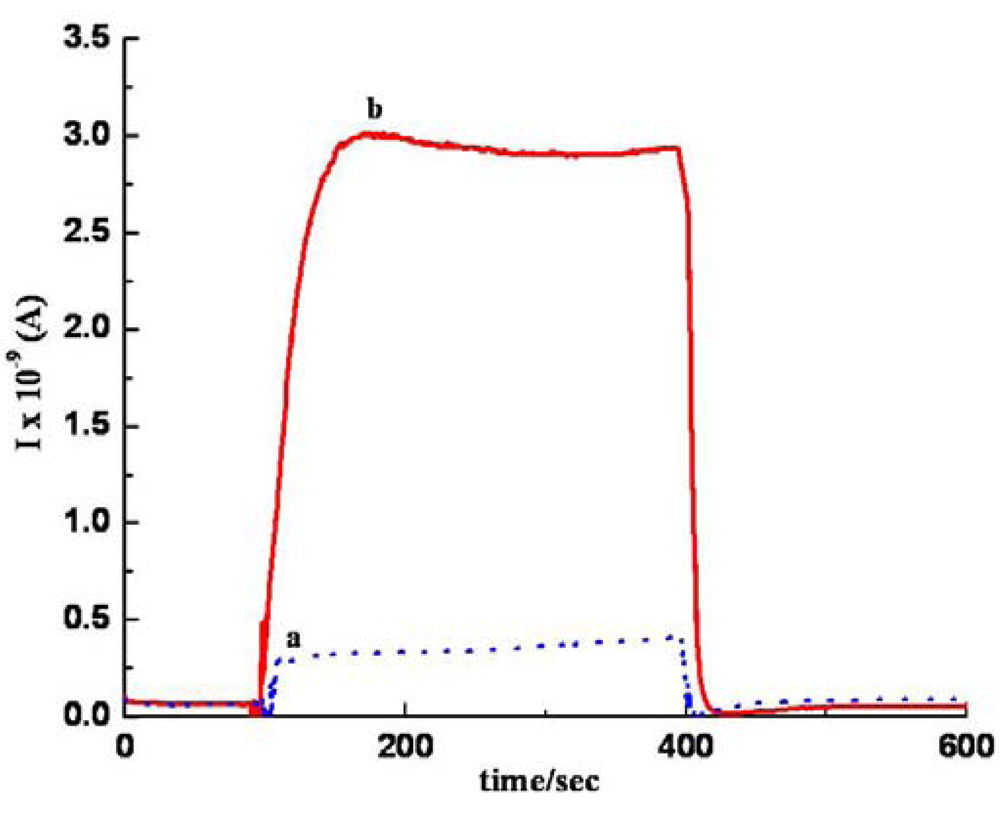
3. Results and Discussion
3.1. Analyte –MWCNTs Interaction
3.2. Analyte - Nylon-6 Interaction
4. Conclusions
Acknowledgments
References
- Li, J.R.; Xu, J.R.; Zhang, M.Q.; Rong, M.Z. Carbon black/polystyrene composites as candidates for gas sensing materials. Carbon 2003, 41(12), 2353–2360. [Google Scholar]
- Sharma, P.; Ahuja, P. Recent advances in carbon nanotube-based electronics. Mater. Res. Bull. 2008, 43(10), 2517–2526. [Google Scholar]
- Liu, A. Towards development of chemosensors and biosensors with metal-oxide-based nanowires or nanotubes. Biosens. Bioelectron. 2008, 24(2), 167–177. [Google Scholar]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nature Mater. 2008, 7(6), 442–453. [Google Scholar]
- Smith, R.G.; D'Souza, N.; Nicklin, S. A review of biosensors and biologically-inspired systems for explosives detection. Analyst 2008, 133(5), 571–584. [Google Scholar]
- Rudnitskaya, A.; Legin, A. Sensor systems, electronic tongues and electronic noses, for the monitoring of biotechnological processes. J. Ind. Microbiol. Biotechnol. 2008, 35(5), 443–451. [Google Scholar]
- Partridge, A.C.; Jansen, M.L.; Arnold, W.M. Conducting polymer-based sensors. Mater. Sci. Eng, C: Biomim. Supramol. Sys. 2000, C12(1-2), 37–42. [Google Scholar]
- Jeong, J.S.; Jeon, S.Y.; Lee, T.Y.; Park, J.H.; Shin, J.H.; Alegaonkar, P.S. Fabrication of MWNTs/nylon conductive composite nanofibers by electrospinning. Dia. Rel. Mat. 2006, 15(11-12), 1839–1843. [Google Scholar]
- Dror, Y.; Salalha, W.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Carbon Nanotubes Embedded in Oriented Polymer Nanofibers by Electrospinning. Langmuir 2003, 19(17), 7012–7020. [Google Scholar]
- Kang, M.; Jin, H.J. Electrospun nanofiber of Nylon 610/multi-walled carbon nanotube composites. Key Eng. Mater. 2006, 321-323, 934–937. [Google Scholar]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45(3), 739–748. [Google Scholar]
- Chen, J.; Tsubokawa, N.; Maekawa, Y.; Yoshida, M. Vapor response properties of conducting composites prepared from crystalline oligomer-grafted carbon black. Carbon 2002, 40(9), 1602–1605. [Google Scholar]
- Sheeney-Haj-Ichia, L.; Basnar, B.; Willner, I. Efficient generation of photocurrents by using CdS/carbon nanotube assemblies on electrodes. Angew. Chem. Int. Ed. 2005, 44(1), 78–83. [Google Scholar]
- Haddon, R.C. Carbon Nanotubes. Acc. Chem. Res. 2002, 35(12), 997. [Google Scholar]
- Sinha, N.; Yeow John, T.W. Carbon nanotubes for biomedical applications. IEEE Trans. Nanobiosci. 2005, 4(2), 180–195. [Google Scholar]
- Niu, C.; Sichel, E.K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70(11), 1480–1482. [Google Scholar]
- Ong, K.G.; Grimes, C.A. A Carbon Nanotube- based Sensor for CO2 Monitoring. Sensors 2001, 1(6), 193–205. [Google Scholar]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuat. B: Chem. 2001, B81(1), 32–41. [Google Scholar]
- Zhang, W.D.; Shen, L.; Phang, I.Y.; Liu, T. Carbon nanotubes reinforced nylon-6 composite prepared by simple melt-compounding. Macromolecules 2004, 37(2), 256–259. [Google Scholar]
- Nadler, M.; Mahrholz, T.; Riedel, U.; Schilde, C.; Kwade, A. Preparation of colloidal carbon nanotube dispersions and their characterisation using a disc centrifuge. Carbon 2008, 46(11), 1384–1392. [Google Scholar]
- Olson, B.G.; Decker, J.J.; Nazarenko, S.; Yudin, V.E.; Otaigbe, J.U.; Korytkova, E.N. Aggregation of Synthetic Chrysotile Nanotubes in the Bulk and in Solution Probed by Nitrogen Adsorption and Viscosity Measurements. J. Phys. Chem C. 2008, 112(33), 12943–12950. [Google Scholar]
- Tang, X.J.; Zhang, G.; Zhao, Y.P. Electrochemical characterization of silver nanorod electrodes prepared by oblique angle deposition. Nanotechnology 2006, 17(17), 4439–4444. [Google Scholar]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Letters 2004, 4(3), 491–496. [Google Scholar]
- Ueda, T.; Katsuki, S.; Takahashi, K.; Narges, H.A.; Ikegami, T.; Mitsugi, F. Fabrication and characterization of carbon nanotube based high sensitive gas sensors operable at room temperature. Dia. Rel. Mat. 2008, 17(7-10), 1586–1589. [Google Scholar]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun Nanofibers in Energy and Environmental Applications. Energy Env. Sci. 2008, 1(2), 205–221. [Google Scholar]
- Thavasi, V.; Jose, R.; Ramakrishna, S. Controlled electron injection and transport at materials interfaces in dye sensitized solar cells. Mat. Sci. Eng. R: Report 2008. [Google Scholar] [CrossRef]
- Rojas, R.; Pinto, N.J. Using electrospinning for the fabrication of rapid response gas sensors based on conducting polymer nanowires. IEEE Sensors J. 2008, 8(6), 951–953. [Google Scholar]
- Pinto, N.J.; Ramos, I.; Rojas, R.; Wang, P.C.; Johnson, AT, Jr. Electric response of isolated electrospun polyaniline nanofibers to vapors of aliphatic alcohols. Sens. Actuat. B: Chem. 2008, B129(2), 621–627. [Google Scholar]
- Shaffer, M.S.P.; Windle, A.H. Fabrication and characterization of carbon nanotube/poly(vinyl alcohol) composites. Adv. Mater. 1999, 11(11), 937–941. [Google Scholar]
- Gou, J.; Minaie, B.; Wang, B.; Liang, Z.; Zhang, C. Computational and experimental study of interfacial bonding of single-walled nanotube reinforced composites. Comp. Mat. Sci. 2004, 31(3-4), 225–236. [Google Scholar]
- Guo, Y.; Wu, J.; Zhang, Y. Manipulation of single-wall carbon nanotubes into aligned molecular layers. Chem. Phys. Lett. 2002, 362(3-4), 314–318. [Google Scholar]
- Guo, Y.; Minami, N.; Kazaoui, S.; Peng, J.; Yoshida, M.; Miyashita, T. Multi-layer LB films of single-wall carbon nanotubes. Physica B: Conden Matter. 2002, 323(1-4), 235–236. [Google Scholar]
- Correa-Duarte, M.A.; Kosiorek, A.; Kandulski, W.; Giersig, M.; Liz-Marzan, L.M. Layer-by-Layer Assembly of Multiwall Carbon Nanotubes on Spherical Colloids. Chem. Mater. 2005, 17(12), 3268–3272. [Google Scholar]
- Sandler, J.; Shaffer, M.S.P.; Prasse, T.; Bauhofer, W.; Schulte, K.; Windle, A.H. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer 1999, 40(21), 5967–5971. [Google Scholar]
- Ericson, L.M. Macroscopic, Neat, Single-Walled Carbon Nanotube Fibers. Science 2004, 305(5689), 1447–1450. [Google Scholar]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polymer composites. Appl. Phys. Lett. 2000, 76(20), 2868–2870. [Google Scholar]
- Paloniemi, H.; Lukkarinen, M.; Aeaeritalo, T.; Areva, S.; Leiro, J.; Heinonen, M. Layer-by-layer electrostatic self-assembly of single-wall carbon nanotube polyelectrolytes. Langmuir 2006, 22(1), 74–83. [Google Scholar]
- Kim, Y.; Lee, DY.; Lee, M.H.; Cho, N.I.; Song, Y.S.; Lee, S.J. Characterization of electrospun ZnO nanofibers. J. Korean Phys. Soc. 2008, 53, 421–425. [Google Scholar]
- Kim, H.S.; Jin, H.J.; Myung, S.J.; Kang, M.; Chin, I.J. Macromol. Rapid Comm. 2006, 27(2), 146–151.
- Kang, M.; Jin, H.J. Electrically conducting electrospun silk membranes fabricated by adsorption of carbon nanotubes. Colloid Polym. Sci. 2007, 285(10), 1163–1167. [Google Scholar]
- Yoon, S.H.; Jin, H.J.; Kook, M.C.; Pyun, Y.R. Electrically conductive bacterial cellulose by incorporation of carbon nanotubes. Biomacromolecules. 2006, 7(4), 1280–1284. [Google Scholar]
- Saran, N.; Parikh, K.; Suh, D.S.; Munoz, E.; Kolla, H.; Manohar, S.K. Fabrication and characterization of thin films of single-walled carbon nanotube bundles on flexible plastic substrates. J. Am. Chem. Soc. 2004, 126(14), 4462–4463. [Google Scholar]
- Gao, J.; Zhao, B.; Itkis, M.E.; Bekyarova, E.; Hu, H.; Kranak, V. Bulk heterojunction conjugated polymers/functionalized single walled carbon nanotubes photovoltaic devices. J. Am. Chem. Soc. 2006, 128(23), 7492–7496. [Google Scholar]
- Huang, J.C. Carbon Black Filled Polymers and Polymer Blends. Adv. Polym. Technol. 2002, 21(4), 299–313. [Google Scholar]
- Philip, B.; Abraham, J.K.; Chandrasekhar, A.; Varadan, V.K. Temperature-compensated strain measurement using fiber Bragg grating sensors embedded in composite laminates. Smart Mater. Struct. 2003, 12(6), 935–939. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K. Nanotube molecular wires as chemical sensors. Science 2000, 287(5453), 622–625. [Google Scholar]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic. vapor detection. Nano Letters 2003, 3(7), 929–933. [Google Scholar]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Polyaniline with fluoroalcohol additives as hydrazine sensors. Polym. Preprints 2005, 46(1), 538–539. [Google Scholar]
- Santos, D.P.; Zanoni, M.V.B.; Bergamini, M.F.; Chiorcea-Paquim, A.M.; Diculescu, V.C.; Oliveira Brett, A.M. Poly(glutamic acid) nanofibre modified glassy carbon electrode: Characterization by atomic force microscopy, voltammetry and electrochemical impedance. Electrochim. Acta 2008, 53(11), 3991–4000. [Google Scholar]
- Baker, S.; Lee, C.S.; Marcus, M.S.; Yang, W; Eriksson, M.; Hamers, R.J. Vertically-aligned carbon nanofibers as electrode materials for biosensing arrays. Abstracts of Papers. 229th ACS National Meeting, San Diego, CA, United States, March 2005; pp. 13–17.
- Matthews, K.; Cruden Brett, A.; Chen, B.; Meyyappan, M.; Delzeit, L. Plasma-enhanced chemical vapor deposition of multiwalled carbon nanofibers. J. Nanosci. Nanotechnol. 2002, 2(5), 475–480. [Google Scholar]











| Analytes | Nature of Analytes | Dipole moment (D) | Vapor pressure kPa @ 25°C | Responsiveness |
|---|---|---|---|---|
| Acetone | Polar | 2.91 | 30.8 | 28.70 |
| Ethyl acetate (EA) | 1.88 | 10.13 | 4.90 | |
| Dichloromethane (DCM) | 1.60 | 58.2 | 11.68 | |
| Trichloromethane (TCM) | 1.08 | 26.2 | 1.48 | |
| Tetrahydrofuran (THF) | 1.63 | 21.6 | 1.38 | |
| Ethanol | 1.60 | 7.87 | 4.34 | |
| Hexane | Non polar | 0.08 | 20.2 | 3.55 |
| Toluene | 0.31 | 3.79 | 0.13 | |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lala, N.L.; Thavasi, V.; Ramakrishna, S. Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability. Sensors 2009, 9, 86-101. https://doi.org/10.3390/s90100086
Lala NL, Thavasi V, Ramakrishna S. Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability. Sensors. 2009; 9(1):86-101. https://doi.org/10.3390/s90100086
Chicago/Turabian StyleLala, Neeta L., Velmurugan Thavasi, and Seeram Ramakrishna. 2009. "Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability" Sensors 9, no. 1: 86-101. https://doi.org/10.3390/s90100086
APA StyleLala, N. L., Thavasi, V., & Ramakrishna, S. (2009). Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability. Sensors, 9(1), 86-101. https://doi.org/10.3390/s90100086






