A Galvanic Sensor for Monitoring the Corrosion Condition of the Concrete Reinforcing Steel: Relationship Between the Galvanic and the Corrosion Currents
Abstract
:1. Introduction
2. Experimental
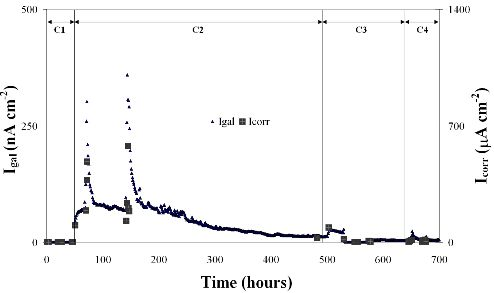
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Gurusamy, K.; Geoghegan, M. Corrosion of Reinforcement in Concrete; Page, C.L., Tredaway, K., Bamforth, B.P., Eds.; SCI, Elsevier Applied Science: New York, NY, USA, 1990; p. 333. [Google Scholar]
- Schießl, P.; Raupach, M. Monitoring system for the corrosion risk of steel in concrete structures. Concr. Int. 1992, 7, 52–55. [Google Scholar]
- Raupach, M. Chloride-induced macrocell corrosion of steel in concrete—theoretical background and practical consequences. Constr. Build. Mater. 1996, 10, 329–338. [Google Scholar]
- Climent-Llorca, M.A.; Viqueira-Pèrez, E.; López-Atalaya, M.M. Embeddable Ag/AgCl sensors for in-situ monitoring chloride contents in concrete. Cem. Concr. Res. 1996, 26, 1157. [Google Scholar]
- Pereira, E. Corrosion Monitoring in Reinforced Concrete. PhD Thesis, University of Lisboa, Lisbon, Portugal, 2004. [Google Scholar]
- Montemor, F.; Alves, J.; Simões, A.; Fernandes, J.; Lourenço, Z.; Costa, A.; Appleton, A.; Ferreira, M.G. Multiprobe chloride sensor for in-situ monitoring of reinforced concrete structures. Cem. Concr. Compos. 2006, 28, 233–236. [Google Scholar]
- Schiessl, P. Effectiveness of coatings. Proceedings of International Conference on Repair of Concrete Structures – From theory to practice in a marine environment, Svolver, Norway, May 20–30, 1997; p. 433.
- Correia, M.J.; Pereira, E.V.; Salta, M.M.; Fonseca, I. Sensor for oxygen evaluation in concrete. Cem. Concr. Compos. 2006, 28, 226–232. [Google Scholar]
- Martinez, I.; Andrade, C. Example of reinforcement corrosion monitoring by embedded sensors in concrete structures. Cem. Concr. Compos. 2009, 31, 454–554. [Google Scholar]
- Bassler, R.; Mietz, J.; Raupach, M.; Klinghoffer, O. Corrosion monitoring sensors for durability assessment of reinforced concrete structures. Proc. SPIE 2000, 3988, 32–39. [Google Scholar]
- Broomfiled, J.; Davies, K.; Hladky, K. The use of permanent corrosion monitoring in new and existing reinforced concrete structures. Cem. Concr. Compos. 2002, 24, 27–34. [Google Scholar]
- Kumar, K.; Muralidharan, S.; Manjula, T.; Karthikeyan, M.S.; Palaniswamy, N. Sensor systems for corrosion monitoring in concrete structures. Sens. Trans. Mag. 2006, 67, 553–560. [Google Scholar]
- Elsener, B. Corrosion rate on reinforced concrete structures determined by electrochemical methods. Mater. Sci. Forum 1995, 192–194, 857–866. [Google Scholar]
- Yoo, J.H.; Park, Z.T.; Kim, J.G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structure: Part 2. Laboratory electrochemical testing of sensors in concrete. Cem. Concr. Res. 2003, 33, 2057–2062. [Google Scholar]
- McCarter, W.J.; Vennesland, O. Sensor system for use in reinforced concrete structures. Constr. Build. Mater. 2004, 18, 351–358. [Google Scholar]
- Song, H.W.; Saraswathy, V. Corrosion monitoring of reinforced concrete structures — a review. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar]
- Islam, M.; Daily, S.F. Use of Electrochemical techniques to assess performance of corrosion protection systems for reinforced concrete structures — a review. J. ASTM Int. 2006, 3, 1546–1557. [Google Scholar]
- Leelalerkiet, V.; Shimizu, T.; Tomoda, Y.; Ohtsu, M. Estimation of corrosion in reinforced concrete by electrochemical techniques and acoustic emission techniques. J. Adv. Concr. Technol. 2005, 3, 137–147. [Google Scholar]
- Smulko, J.M.; Darowicki, K.; Zieliñski, A. Evaluation of reinforcement corrosion rate in concrete structures by electrochemical noise measurements. Russ. J. Electrochem. 2006, 42, 548–552. [Google Scholar]
- Andrade, C.; Alonso, C. Corrosion monitoring in the laboratory and on–site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar]
- Zheng, Z.; Sun, X.; Lei, Y. Monitoring corrosion of reinforcement in concrete structures via fiber Bragg grating sensors. Front. Mech. Eng. China 2009, 4, 316–319. [Google Scholar]
- Dickerson, N.; Simonen, J.; Andriga, M.; Wood, S.; Neikirk, D. Wireless low-cost corrosion sensors for reinforced concrete structures. Proc. SPIE 2005, 5765, 1166. [Google Scholar]
- Christensen, P.T. Stochastic modeling of the crack initiation time for reinforced concrete structures. Proceedings of ASCE 2000 Structures Congress,, Philadelphia, PA, USA, USA, May 8–10, 2000.
- Park, T.; Choy, Y.S.; Kim, J.G.; Chung, L. Development of a galvanic sensor for detecting the corrosion damage of the steel embedded in concrete structures. Part 2. Laboratory electrochemical testing of sensors in concrete. Cem. Concr. Res. 2005, 35, 1814–1819. [Google Scholar]
- Figueira, R.; Pereira, E.V.; Salta, M.M.; Fonseca, I.T.E. Long-term efficiency of two organic inhibitors for reinforced concrete. Mater. Sci. Forum 2008, 587–588, 677–681. [Google Scholar]
- Raupach, M. Models for the propagation phase of reinforcement corrosion - an overview. Mater. Corros. 2006, 57, 605–613. [Google Scholar]






| Elements in % | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | V | W | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stainless steel | 0.03 | 0.4 | 2 | 0.03 | 0.03 | 17 | 2 | 11 | 0.5 | 0.06 | 0.03 | 0.05 | <68 |
| Carbon steel | 0.1 | 0.2 | 0.6 | 0.02 | 0.03 | 0.1 | 0.02 | 0.2 | 0.5 | 0.002 | 0.02 | 0.02 | 98 |
| Steel condition | Icorr (μA cm−2) | Igal (nA cm−2) |
|---|---|---|
| passive state | <0.1 | <0.14 |
| high active corrosion | >1 | >1 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, E.V.; Figueira, R.B.; Salta, M.M.L.; Da Fonseca, I.T.E. A Galvanic Sensor for Monitoring the Corrosion Condition of the Concrete Reinforcing Steel: Relationship Between the Galvanic and the Corrosion Currents. Sensors 2009, 9, 8391-8398. https://doi.org/10.3390/s91108391
Pereira EV, Figueira RB, Salta MML, Da Fonseca ITE. A Galvanic Sensor for Monitoring the Corrosion Condition of the Concrete Reinforcing Steel: Relationship Between the Galvanic and the Corrosion Currents. Sensors. 2009; 9(11):8391-8398. https://doi.org/10.3390/s91108391
Chicago/Turabian StylePereira, Elsa Vaz, Rita Bacelar Figueira, Maria M. Lemos Salta, and Inês T Elias Da Fonseca. 2009. "A Galvanic Sensor for Monitoring the Corrosion Condition of the Concrete Reinforcing Steel: Relationship Between the Galvanic and the Corrosion Currents" Sensors 9, no. 11: 8391-8398. https://doi.org/10.3390/s91108391
APA StylePereira, E. V., Figueira, R. B., Salta, M. M. L., & Da Fonseca, I. T. E. (2009). A Galvanic Sensor for Monitoring the Corrosion Condition of the Concrete Reinforcing Steel: Relationship Between the Galvanic and the Corrosion Currents. Sensors, 9(11), 8391-8398. https://doi.org/10.3390/s91108391