Resistive Oxygen Sensor Using Ceria-Zirconia Sensor Material and Ceria-Yttria Temperature Compensating Material for Lean-Burn Engine
Abstract
:1. Introduction
2. Experimental
2.1. Sample preparation
2.1.1. Ceria-yttria powders
2.1.2. Ceria-zirconia powder
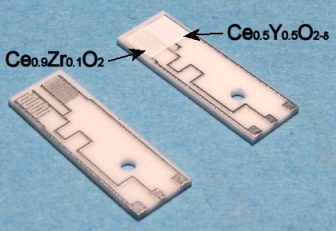
2.1.3. Thick films
2.1.4. Electrodes
2.2. Evaluation
3. Results and Discussion
3.1. Optimization of Y concentration
3.2. Sensor element
4. Conclusions
References and Notes
- Ivers-Tiffee, E.; Hardtl, K.H.; Menesklou, W.; Riegel, J. Principles of solid state oxygen sensors for lean combustion gas control. Electrochim. Acta 2001, 47, 807–814. [Google Scholar]
- Rettig, F.; Moos, R.; Plog, C. Poisoning of temperature independent resistive oxygen sensors by sulfur dioxide. J. Electroceram. 2004, 13, 733–738. [Google Scholar]
- Sahner, K.; Moos, R.; Izu, N.; Shin, W.; Murayama, N. Response kinetics of temperature-independent resistive oxygen sensor formulations: a comparative study. Sens. Actuat. B-Chem. 2006, 113, 112–119. [Google Scholar]
- Neri, G.; Micali, G.; Bonavita, A.; Licheri, R.; Orru, R.; Cao, G.; Marzorati, D.; Borla, E.M.; Roncari, E.; Sanson, A. FeSrTiO3-based resistive oxygen sensors for application in diesel engines. Sens. Actuat. B-Chem. 2008, 134, 647–653. [Google Scholar]
- Moos, R.; Rettig, F.; Hurland, A.; Plog, C. Temperature-independent resistive oxygen exhaust gas sensor for lean-burn engines in thick-film technology. Sens. Actuat. B-Chem. 2003, 93, 43–50. [Google Scholar]
- Izu, N.; Shin, W.; Murayarna, N.; Kanzaki, S. Resistive oxygen gas sensors based on CeO2 fine powder prepared using mist pyrolysis. Sens. Actuat. B-Chem. 2002, 87, 95–98. [Google Scholar]
- Izu, N.; Shin, W.; Murayama, N. Fast response of resistive-type oxygen gas sensors based on nano-sized ceria powder. Sens. Actuat. B-Chem. 2003, 93, 449–453. [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Small temperature-dependent resistive oxygen gas sensors using Ce0.9Y0.1O2-δ as a new temperature compensating material. Sens. Actuat. B-Chem. 2004, 101, 381–386. [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Evaluation of response characteristics of resistive oxygen sensors based on porous cerium oxide thick film using pressure modulation method. Sens. Actuat. B-Chem. 2006, 113, 207–213. [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Improvement in response of resistive oxygen sensor based on ceria-zirconia thick film with Pt catalyst on surface. Sens. Actuat. B-Chem. 2009, 139, 317–321. [Google Scholar]
- Varhegyi, E.B.; Perczel, I.V.; Gerblinger, J.; Fleischer, M.; Meixner, H.; Giber, J. Auger and sims study of segregation and corrosion behavior of some semiconducting oxide gas-sensor materials. Sens. Actuat. B-Chem. 1994, 19, 569–572. [Google Scholar]
- Varhegyi, E.B.; Gerblinger, J.; Reti, F.; Perczel, I.V.; Meixner, H. Study of the behavior of CeO2 in SO2-containing environment. Sens. Actuat. B-Chem. 1995, 25, 631–635. [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Influence of SO2 gas on output of resistive oxygen sensors using CeO2 and Ce0.8Zr0.2O2. J. Electrochem. Soc. 2005, 152, H111–H114. [Google Scholar]
- Izu, N.; Oh-hori, N.; Itou, M.; Shin, W.; Matsubara, I.; Murayama, N. Resistive oxygen gas sensors based on Ce1-xZrxO2 nano powder prepared using new precipitation method. Sens. Actuat. B-Chem. 2005, 108, 238–243. [Google Scholar]
- Esper, M.J.; Logothetis, E.M.; Chu, J.C. Titania exhaust gas sensor for automotive applications. SAE Tech. Paper 1979. No. 790140. [Google Scholar]
- Fleischer, M.; Hanrieder, W.; Meixner, H. Oxygen sensor with semiconducting gallium oxide. European Patent EP0,464,243, 1995. [Google Scholar]
- Fleischer, M.; Meixner, H. Sensor for detecting reducing gases. European Patent EP0,464,244, 1992. [Google Scholar]
- Gerblinger, J.; Meixner, H. Gas and temp. sensor arrangement — for temp. independent determination of esp. oxygen at partial pressure. German Patent DE4,210,397, 1993. [Google Scholar]
- Gerblinger, J.; Meixner, H. Device for determining the partial pressure of gases in a gas mixture. European Patent EP0,563,613, 1993. [Google Scholar]
- Sugie, J. Oxide semiconductor gas sensor. Japanese Patent H06-222,026, 1994. [Google Scholar]
- Yahiro, H.; Baba, Y.; Eguchi, K.; Arai, H. High-temperature fuel-cell with ceria-yttria solid electrolyte. J. Electrochem. Soc. 1988, 135, 2077–2080. [Google Scholar]
- Frank, J.; Fleischer, M.; Meixner, H. Gas sensor. World Patent WO9,608,712, 1996. [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N.; Oh-hori, N.; Itou, M. Temperature independent resistive oxygen sensors using solid electrolyte zirconia as a new temperature compensating material. Sens. Actuat. B-Chem. 2005, 108, 216–222. [Google Scholar]
- Izu, N.; Nishizaki, S.; Itoh, T.; Shin, W.; Matsubara, I.; Murayama, N. Output evaluation of resistive oxygen sensor having Ce0.9Zr0.1O2 sensing material and Zr0.8Y0.2O2-δ temperature compensating material in model exhaust gas. J. Ceram. Soc. Jpn. 2007, 115, 688–691. [Google Scholar]
- Sammes, N.M.; Cai, Z.H. Ionic conductivity of ceria/yttria stabilized zirconia electrolyte materials. Solid State Ionics 1997, 100, 39–44. [Google Scholar]
- Dirstine, R.T.; Blumental, R.N.; Kuech, T.F. Ionic conductivity of calcia, yttria, and rare earth-doped cerium dioxide. J. Electrochem. Soc. 1979, 126, 264–269. [Google Scholar]
- Izu, N.; Oh-hori, N.; Shin, W.; Matsubara, I.; Murayama, N.; Itou, M. Response of resistive oxygen sensors using Ce1-xZrxO2 (x = 0.05, 0.10) thick films in propane combustion gas. Sens. Actuat. B-Chem. 2008, 130, 105–109. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar]
- McCullough, J.D.; Britton, J.D. X-ray studies of rare earth oxide systems. II. the oxide systems CeIV-SmIII, CeIV-GdIII, CeIV-YIII, PrIV-YIII and PrIII-YIII. J. Am. Chem. Soc. 1952, 74, 5225–5227. [Google Scholar]
- Longo, V.; Podda, L. Phase-equilibrium diagram of the system ceria-yttria for temperatures between 900-degrees-C and 1700-degrees-C. J. Mater. Sci. 1981, 16, 839–841. [Google Scholar]
- Djurovic, D.; Zinkevich, M.; Aldinger, F. Thermodynamic modeling of the yttrium-oxygen system. CALPHAD 2007, 31, 560–566. [Google Scholar]
- VanHerle, J.; Horita, T.; Kawada, T.; Sakai, N.; Yokokawa, H.; Dokiya, M. Low temperature fabrication of (Y,Gd,Sm)-doped ceria electrolyte. Solid State Ionics 1996, 86, 1255–1258. [Google Scholar]
- Cheng, C.H.; Lee, S.F.; Hong, C.W. Ionic dynamics of an intermediate-temperature yttria-doped-ceria electrolyte. J. Electrochem. Soc. 2007, 154, E158–E163. [Google Scholar]








© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Izu, N.; Nishizaki, S.; Shin, W.; Itoh, T.; Nishibori, M.; Matsubara, I. Resistive Oxygen Sensor Using Ceria-Zirconia Sensor Material and Ceria-Yttria Temperature Compensating Material for Lean-Burn Engine. Sensors 2009, 9, 8884-8895. https://doi.org/10.3390/s91108884
Izu N, Nishizaki S, Shin W, Itoh T, Nishibori M, Matsubara I. Resistive Oxygen Sensor Using Ceria-Zirconia Sensor Material and Ceria-Yttria Temperature Compensating Material for Lean-Burn Engine. Sensors. 2009; 9(11):8884-8895. https://doi.org/10.3390/s91108884
Chicago/Turabian StyleIzu, Noriya, Sayaka Nishizaki, Woosuck Shin, Toshio Itoh, Maiko Nishibori, and Ichiro Matsubara. 2009. "Resistive Oxygen Sensor Using Ceria-Zirconia Sensor Material and Ceria-Yttria Temperature Compensating Material for Lean-Burn Engine" Sensors 9, no. 11: 8884-8895. https://doi.org/10.3390/s91108884
APA StyleIzu, N., Nishizaki, S., Shin, W., Itoh, T., Nishibori, M., & Matsubara, I. (2009). Resistive Oxygen Sensor Using Ceria-Zirconia Sensor Material and Ceria-Yttria Temperature Compensating Material for Lean-Burn Engine. Sensors, 9(11), 8884-8895. https://doi.org/10.3390/s91108884