A Butyl Methacrylate Monolithic Column Prepared In-Situ on a Microfluidic Chip and its Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and instruments
2.2. Microchip Fabrication
2.3. Monolith column polymerization
2.4. Sample analysis process by microfluidic chip
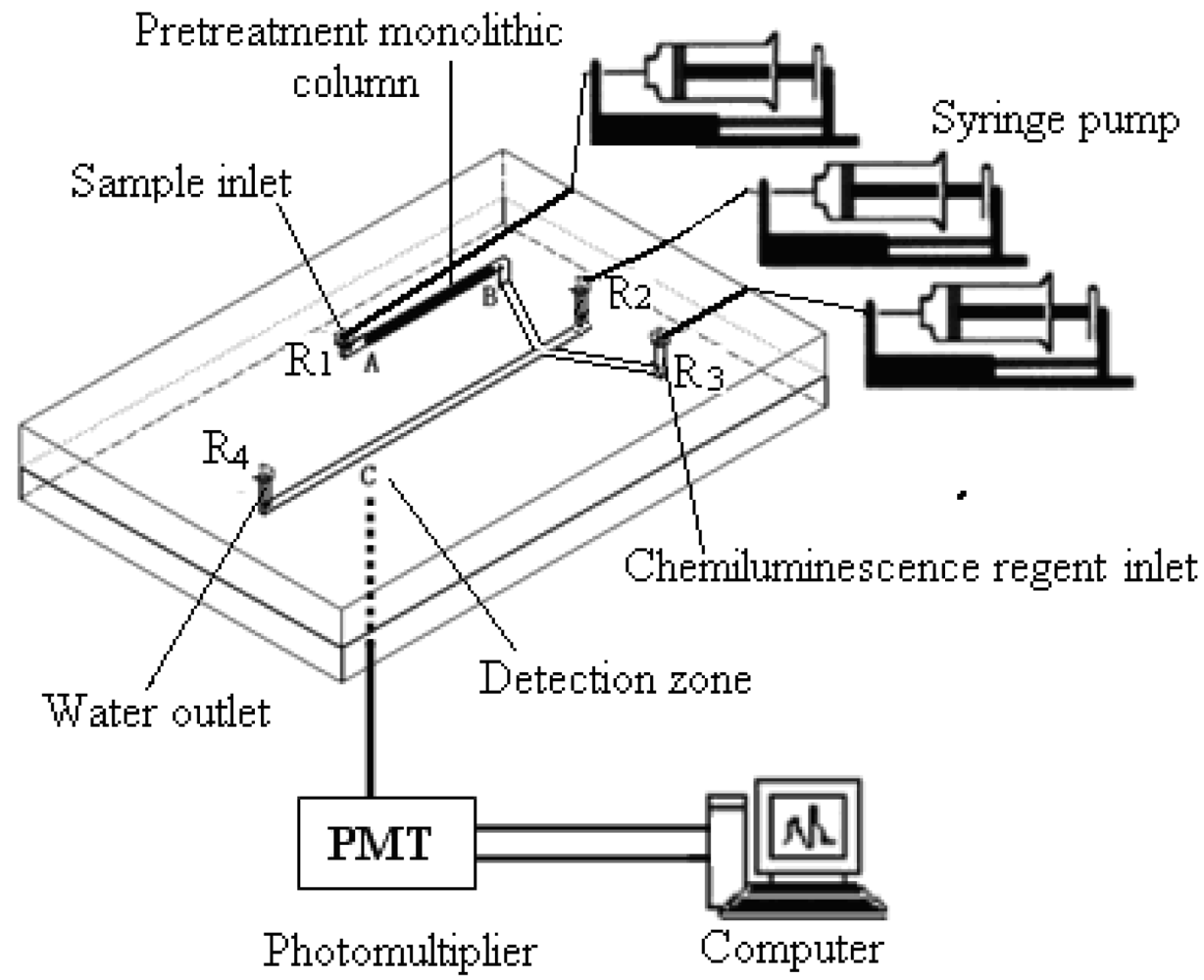
2.4.1. Sample analysis system
2.4.2. Sample loading and elution
3. Results and Discussion
3.1. Microchip Fabrication technology
3.2. Structure and performance of the in-situ polymerized monolith column
3.3. Microfluidic chip analytical system
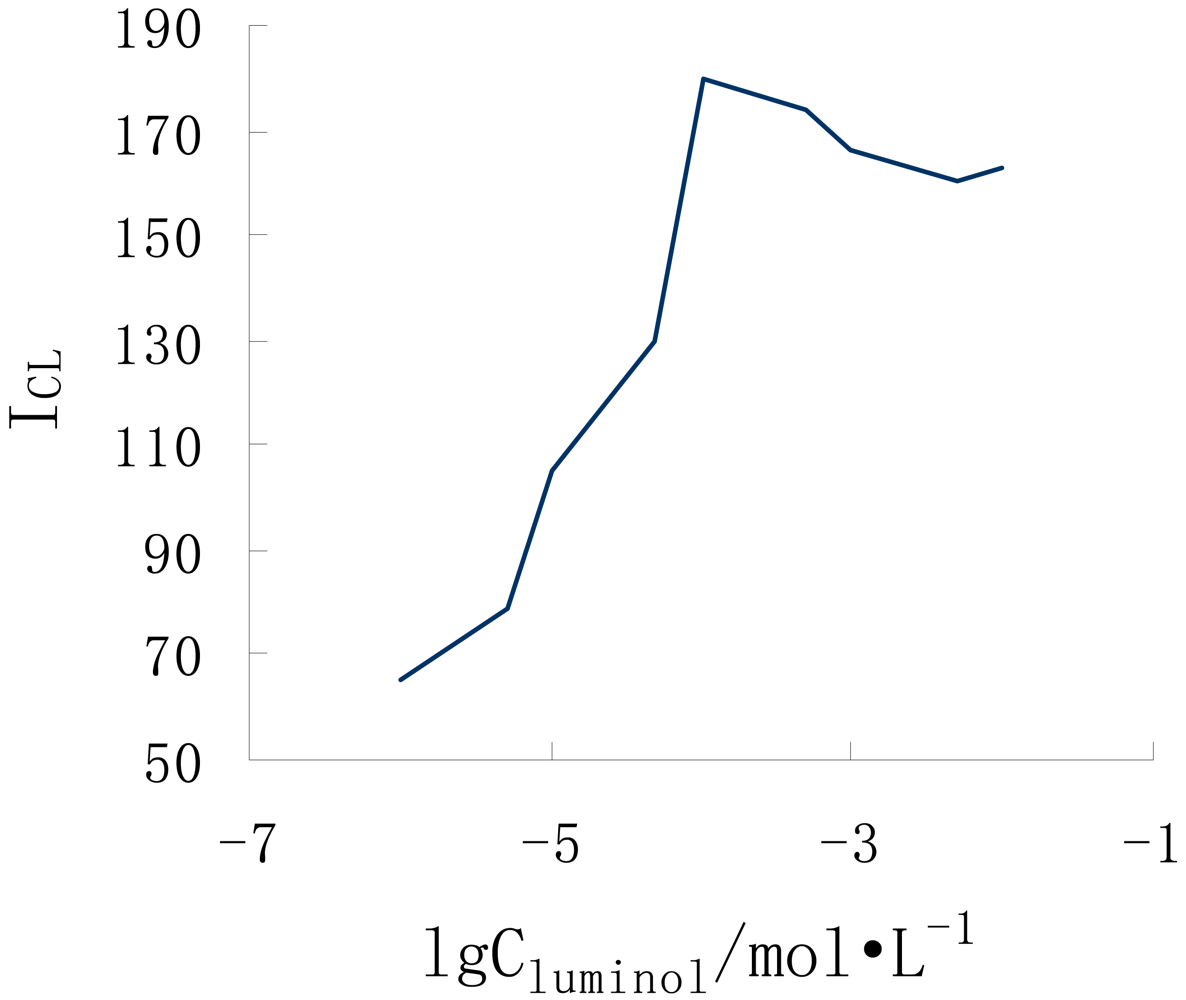
3.4. Promethazine detection by FIA-CL on the microchip
3.5. Sample analysis
4. Conclusions
Acknowledgments
References and Notes
- Yang, Y.; Li, C.; Kameoka., J.; Lee, K.H.; Craighead, H.G. A polymeric microchip with integrated tips and in-situ polymerized monolith for electro-spray mass spectrometry. Lab Chip. 2005, 5, 869–876. [Google Scholar]
- Verpoorte, E. Beads and chips: new recipes for analysis. Lab Chip. 2003, 3, 60N–68N. [Google Scholar]
- Wang, P.C.; DeVoe, D.L.; Lee, C.S. Integration of polymeric membranes with microfluidic networks for bioanalytical applications. Electrophoresis 2001, 22, 3857–3867. [Google Scholar]
- Svec, F.; Tennikova, T.B.; Deyl, Z. Monolithic Material: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2003; pp. 761–773. [Google Scholar]
- Tan, A.; Benetton, S.; Henion, J. D. Chip-Based Solid-Phase Extraction Pretreatment for Direct Electro-spray Mass Spectrometry Analysis Using an Array of Monolithic Columns in a Polymeric Substrate. Anal. Chem. 2003, 75, 5504–5511. [Google Scholar]
- Throckmorton, D.; Shepodd, T.; Singh, A. Electro-chromatography in microchips: reversed-phase separation of peptides and amino acids using photo-patterned rigid polymer monoliths. Anal. Chem. 2002, 74, 784–789. [Google Scholar]
- Ping, G.C.; Yuan, X.L.; Zhang, Y.K. Preparation and Application of Monolithic Column. Chin. J. Anal. Chem. H. 2001, 29, 1464–1469. [Google Scholar]
- Wei, F.; Lin, B.; Feng, Y.Q. Applications of Monoliths in Sample Preconcentration. Chin. J. Chromatogr. H. 2007, 25, 150–156. [Google Scholar]




© 2009 by the authors, licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.; Zhang, W.; Zeng, P.; Cao, Q. A Butyl Methacrylate Monolithic Column Prepared In-Situ on a Microfluidic Chip and its Applications. Sensors 2009, 9, 3437-3446. https://doi.org/10.3390/s90503437
Xu Y, Zhang W, Zeng P, Cao Q. A Butyl Methacrylate Monolithic Column Prepared In-Situ on a Microfluidic Chip and its Applications. Sensors. 2009; 9(5):3437-3446. https://doi.org/10.3390/s90503437
Chicago/Turabian StyleXu, Yi, Wenpin Zhang, Ping Zeng, and Qiang Cao. 2009. "A Butyl Methacrylate Monolithic Column Prepared In-Situ on a Microfluidic Chip and its Applications" Sensors 9, no. 5: 3437-3446. https://doi.org/10.3390/s90503437
APA StyleXu, Y., Zhang, W., Zeng, P., & Cao, Q. (2009). A Butyl Methacrylate Monolithic Column Prepared In-Situ on a Microfluidic Chip and its Applications. Sensors, 9(5), 3437-3446. https://doi.org/10.3390/s90503437



