Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine
Abstract
:1. Introduction
2. Experimental
2.1. Instruments and Reagents
2.2. Preparation of GO/ZIF-67
2.3. Preparing the Modified Electrode
2.4. Preparing the Real Samples
3. Results and Discussion
3.1. Characterizing the GO/ZIF-67 Nanocomposites
3.1.1. Energy-Dispersive X-ray Spectroscopy (EDX)
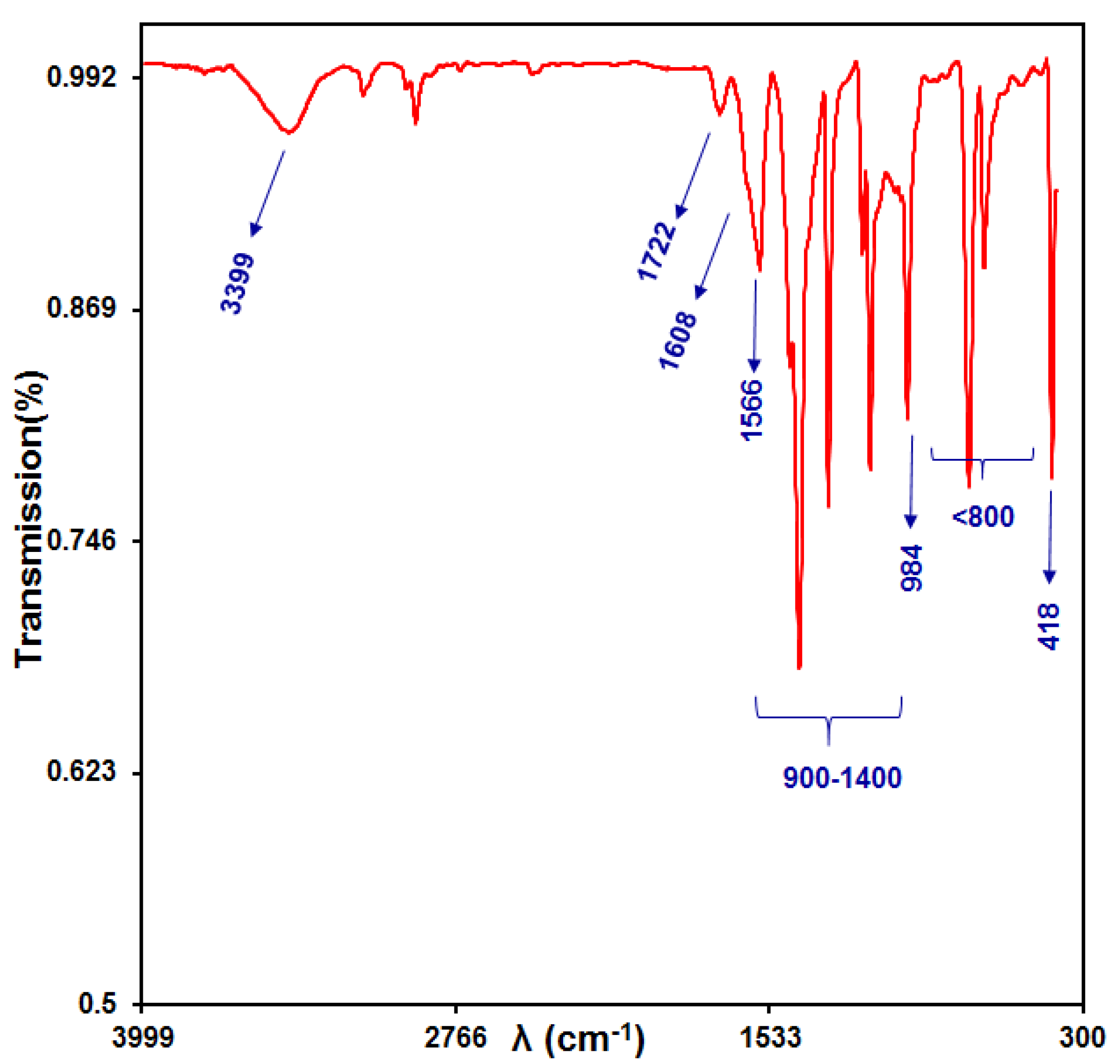
3.1.2. FTIR Analysis
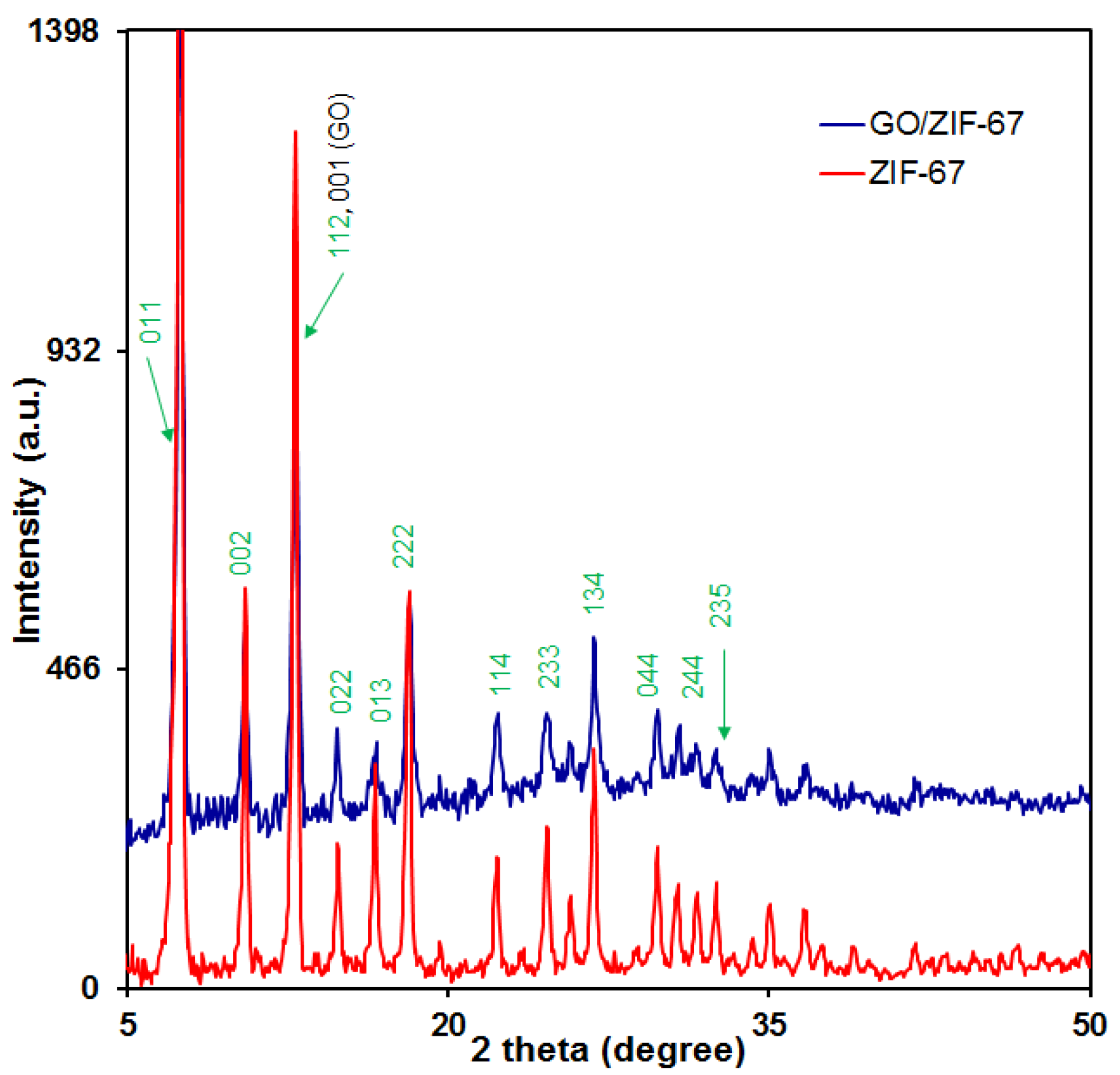
3.1.3. XRD Analysis
3.1.4. FESEM and TEM Spectroscopy
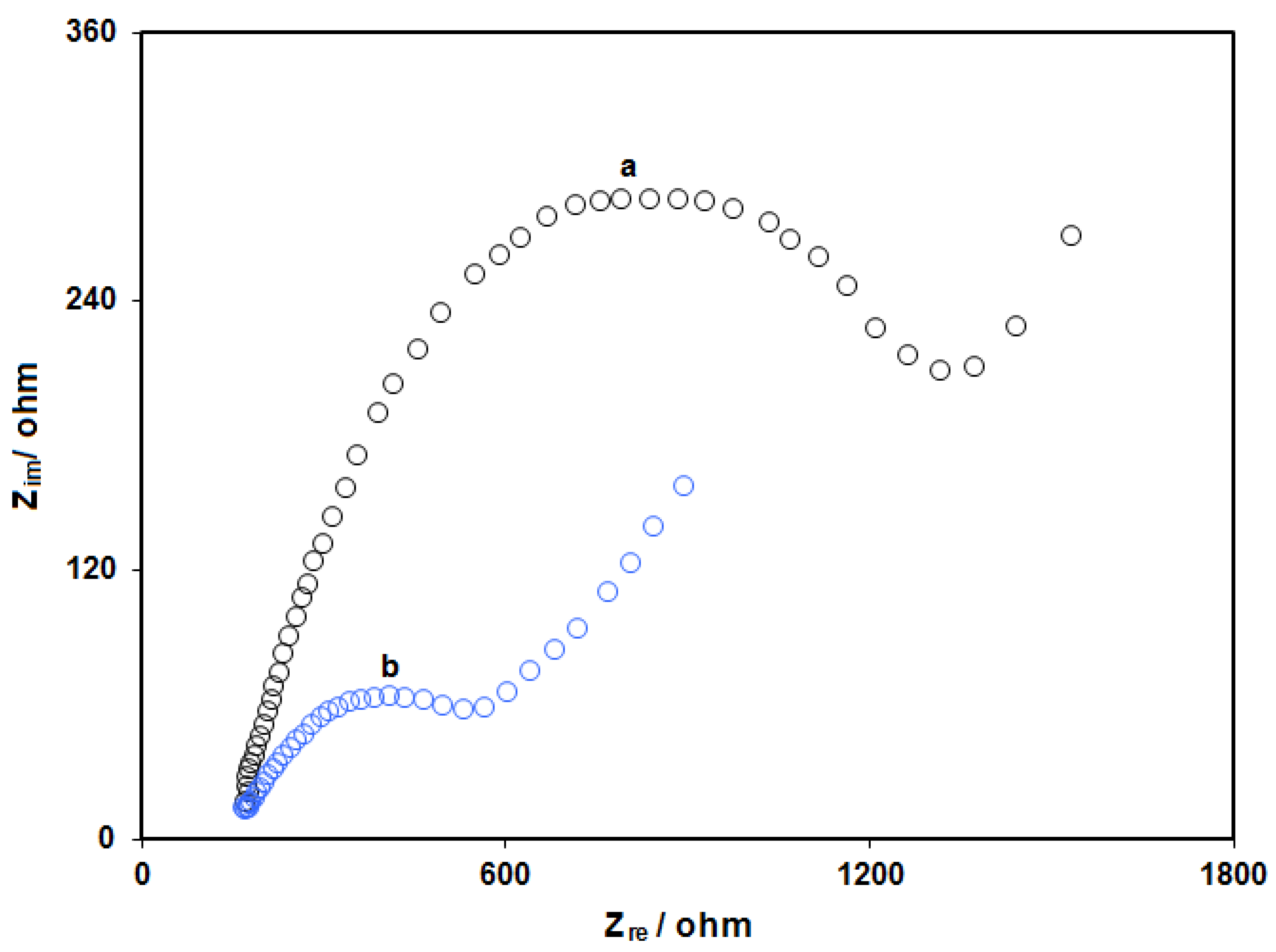
3.1.5. Electrochemical Impedance Spectroscopy (EIS) Studies
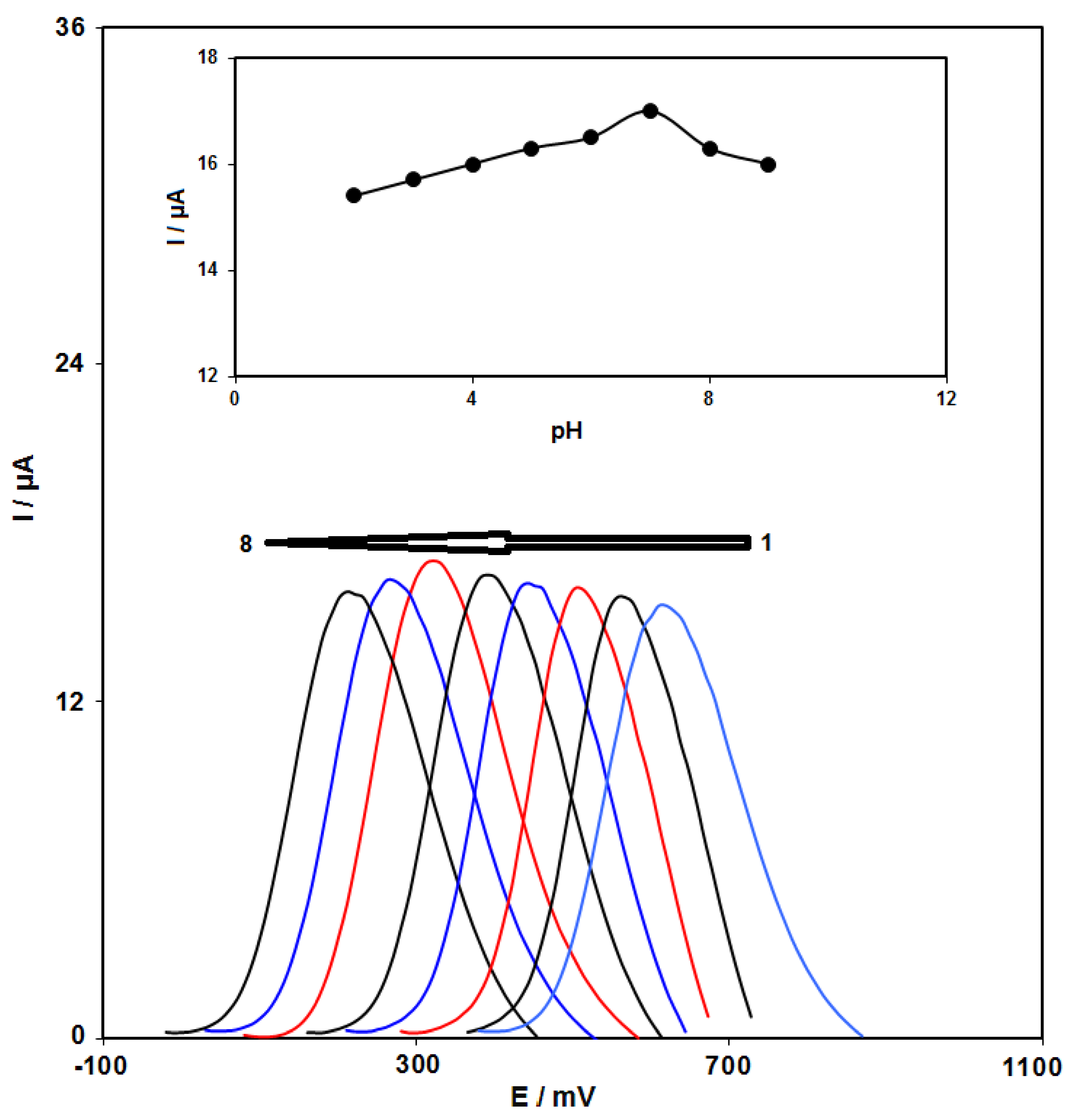
3.2. pH Impact
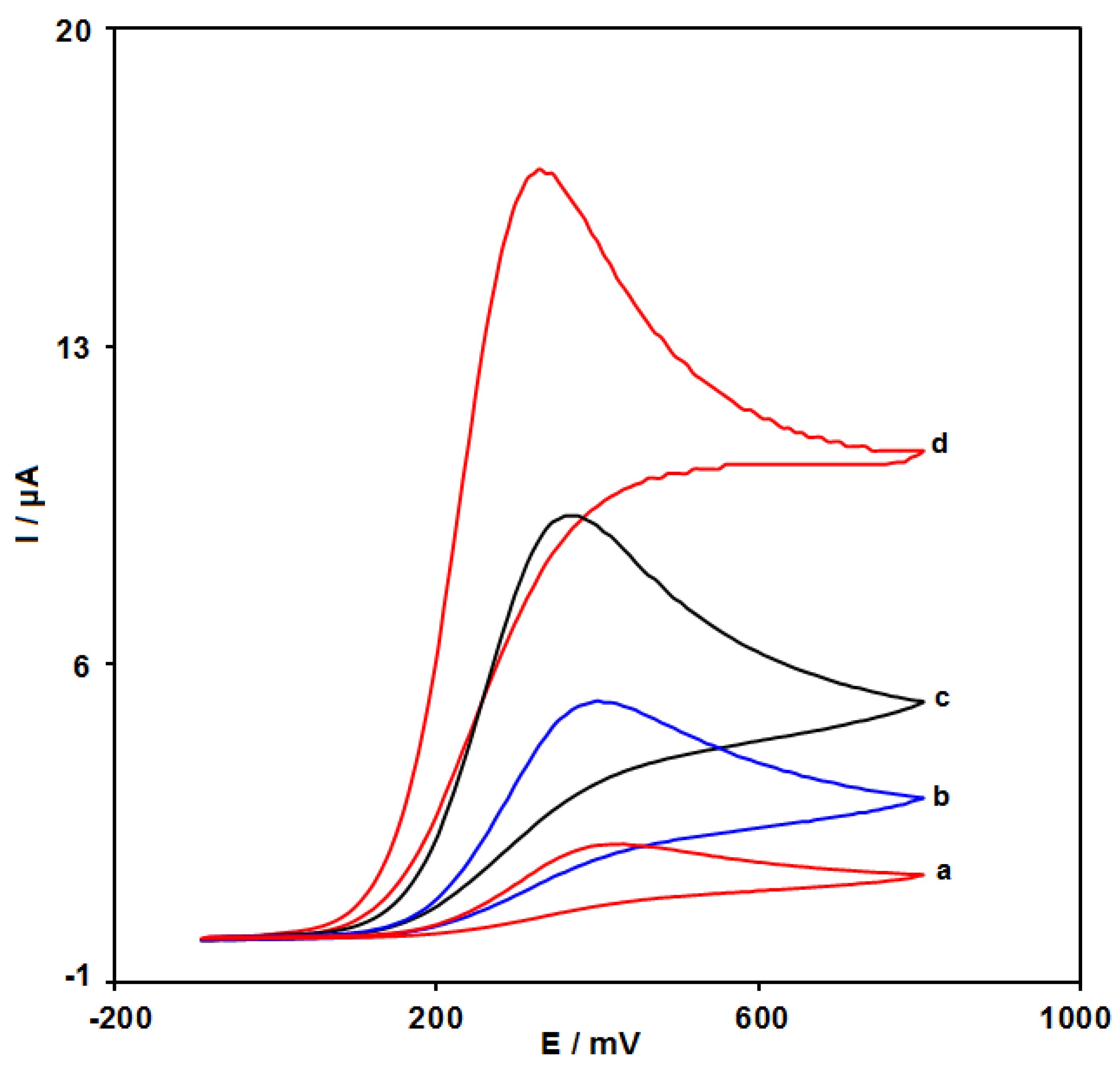
3.3. Electrochemical Behaviors of Epinine at the Surface of Various Electrodes
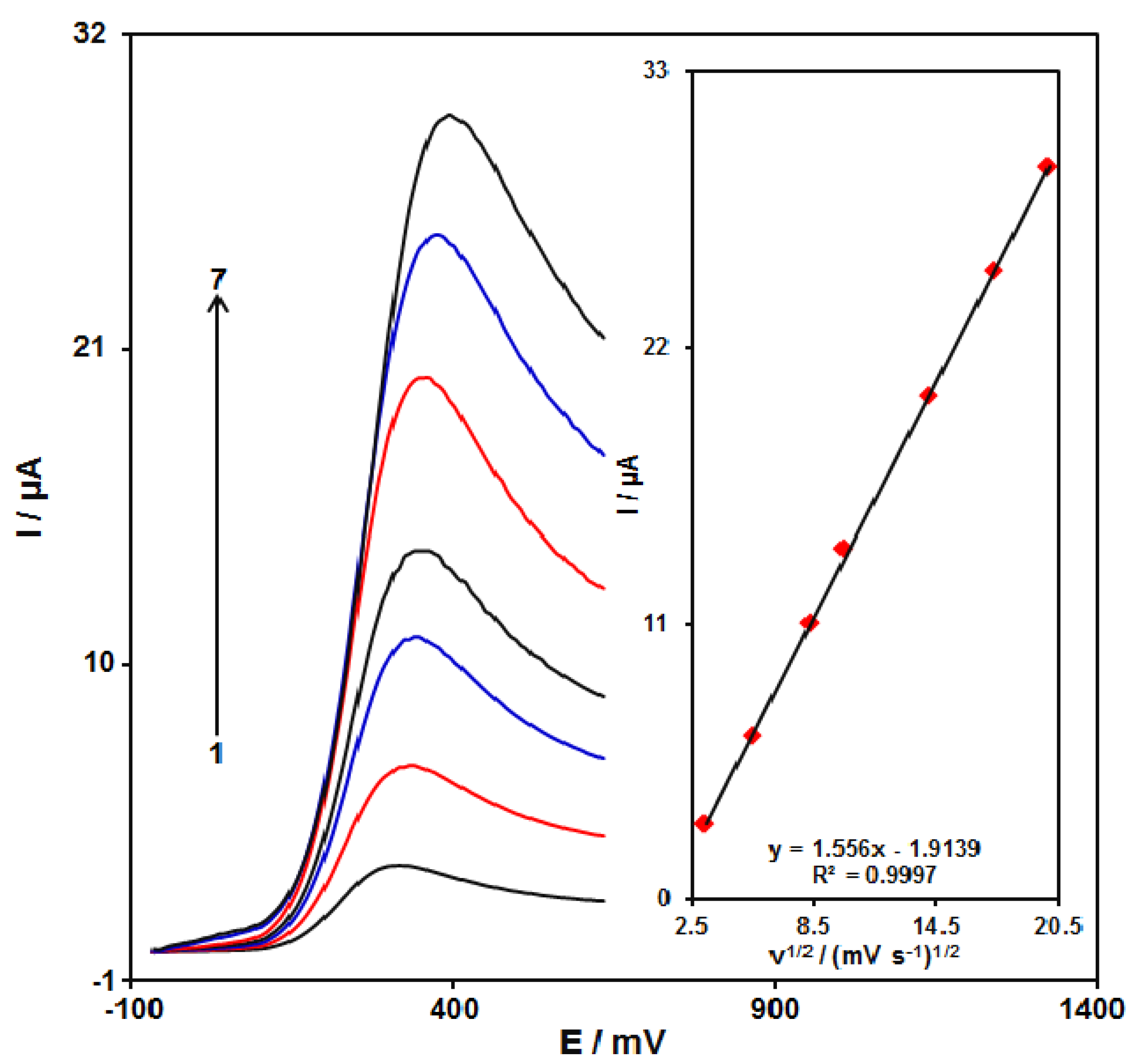
3.4. The Impact of the Potential Scan Rate
3.5. Tafel Analysis
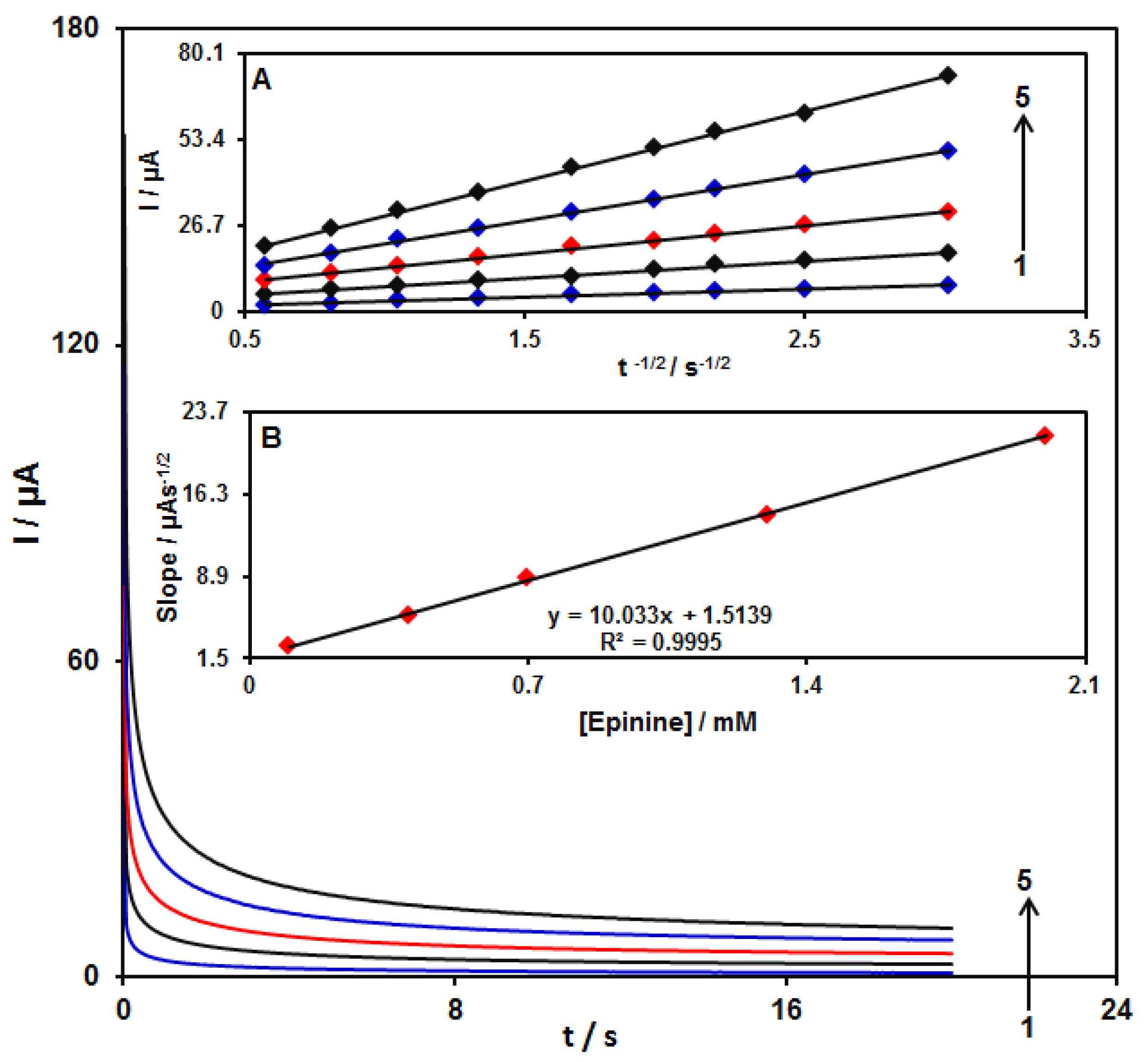
3.6. Chronoamperometric Examinations
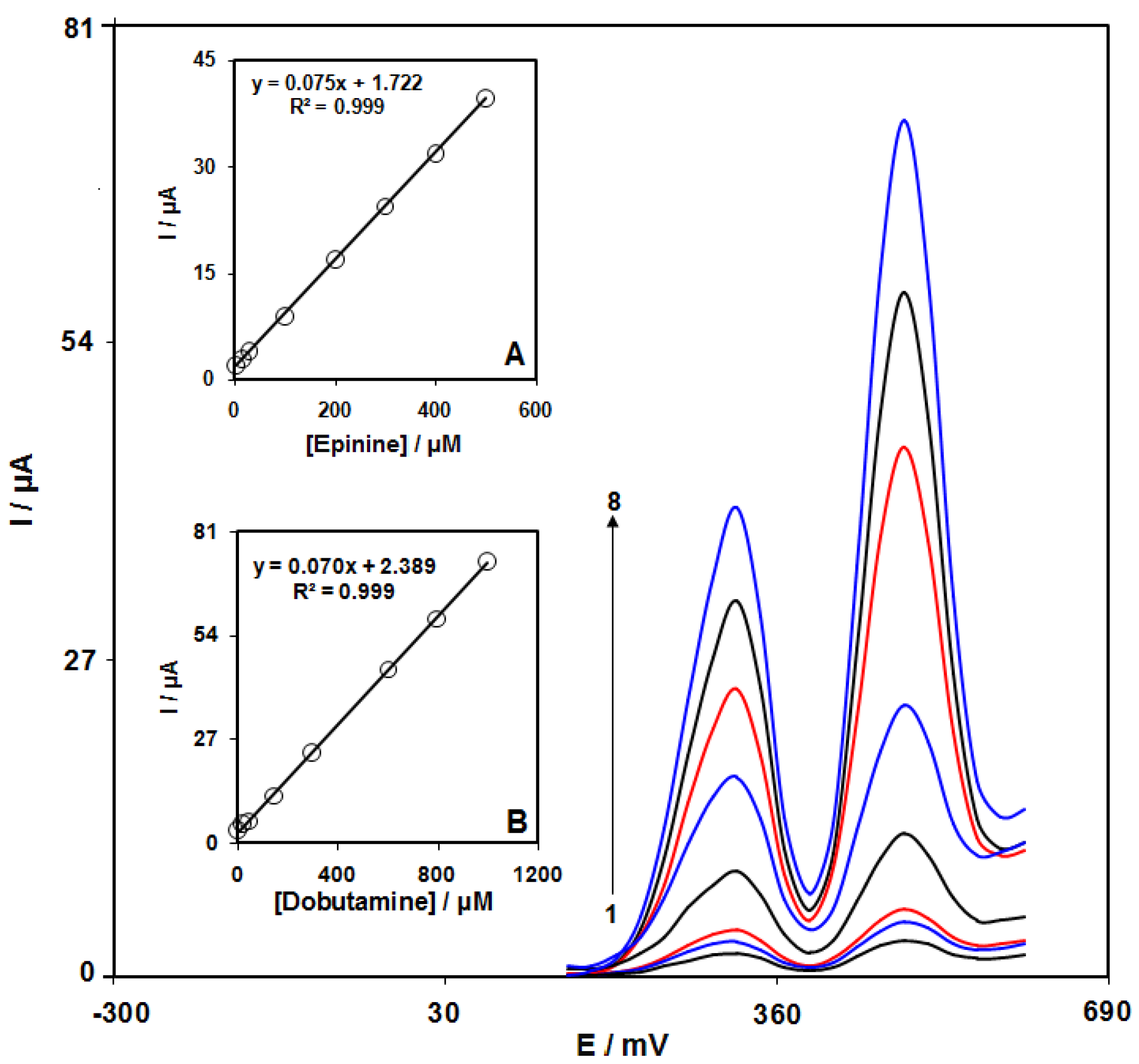
3.7. Determination of the Epinine Voltammetry
3.8. Simultaneous Detection of Epinine and Dobutamine
3.9. Stability and Repeatability of the Modified Electrodes
3.10. Interference Study
3.11. Analysis of the Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lechin, F.; Van der Dijs, B.; Lechin, A.E. Circulating Serotonin, Catecholamines, and Central Nervous System Circuitry Related to Some Cardiorespiratory, Vascular, and Hematological Disorders. J. Appl. Res. 2005, 5, 605–621. [Google Scholar]
- McEwen, B.S. Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metabolism 2003, 52, 10–16. [Google Scholar] [CrossRef]
- Martínez-Mir, I.; Palop, V.; Morales-Olivas, F.; Estañ, L.; Rubio, E. The effects of epinine on arterial blood pressure and regional vascular resistances in anesthetized rats. Gen. Pharmacol. Vasc. Syst. 1998, 31, 75–79. [Google Scholar] [CrossRef]
- Docci, D.; Pistocchi, E.; Turci, F.; Baldrati, L. Effect of ibopamine on the progression of chronic renal failure. Clin. Nephrol. 1986, 26, 121–124. [Google Scholar] [PubMed]
- Carpenter, J.F. An improved synthesis of 5, 6-diacetoxy-N-methylindole and of epinine. J. Org. Chem. 1993, 58, 1607–1609. [Google Scholar] [CrossRef]
- Barger, G.; Dale, H.H. Chemical structure and sympathomimetic action of amines. J. Physiol. 1910, 41, 19–59. [Google Scholar] [CrossRef] [PubMed]
- Tainter, M. Comparative actions of sympathomimetic compounds: Catechol derivatives. J. Pharmacol. Exp. Ther. 1930, 40, 43–64. [Google Scholar]
- Atta, N.F.; Galal, A.; Abdel-Gawad, F.M.; Mohamed, E.F. Electrochemistry and detection of dobutamine at gold nanoparticles cobalt-phthalocyanine modified carbon paste electrode. J. Electrochem. Soc. 2015, 162, B304–B311. [Google Scholar] [CrossRef]
- Al-Hesayen, A.; Azevedo, E.R.; Newton, G.E.; Parker, J.D. The effects of dobutamine on cardiac sympathetic activity in patients with congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Fouladgar, M. Direct voltammetric determination of dobutamine in pharmaceutical samples using multiwall carbon nanotubes paste electrode. ECS Solid State Lett. 2015, 4, M15. [Google Scholar] [CrossRef]
- Keski-Hynnilä, H.; Kurkela, M.; Elovaara, E.; Antonio, L.; Magdalou, J.; Luukkanen, L.; Taskinen, J.; Kostiainen, R. Comparison of electrospray, atmospheric pressure chemical ionization, and atmospheric pressure photoionization in the identification of apomorphine, dobutamine, and entacapone phase II metabolites in biological samples. J. Anal. Chem. 2002, 74, 3449–3457. [Google Scholar] [CrossRef]
- Majerus, T.C.; Dasta, J.F.; Bauman, J.L.; Danziger, L.H.; Ruffolo, R.R., Jr. Dobutamine: Ten years later. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1989, 9, 245–259. [Google Scholar] [CrossRef]
- Whiting, M.J.; Doogue, M.P. Advances in biochemical screening for phaeochromocytoma using biogenic amines. Clin. Biochem. Rev. 2009, 30, 3. [Google Scholar]
- Pussard, E.; Neveux, M.; Guigueno, N. Reference intervals for urinary catecholamines and metabolites from birth to adulthood. Clin. Biochem. 2009, 42, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Kulinskiĭ, V.; Kolesnichenko, L. Catecholamines: Biochemistry, pharmacology, physiology, clinical aspects. Vopr. Med. Khim. 2002, 48, 45–67. [Google Scholar]
- He, H.; Stein, C.M.; Christman, B.; Wood, A.J. Determination of catecholamines in sheep plasma by high-performance liquid chromatography with electrochemical detection: Comparison of deoxyepinephrine and 3, 4-dihydroxybenzylamine as internal standard. J. Chromatogr. B Biomed. Appl. 1997, 701, 115–119. [Google Scholar] [CrossRef]
- Musso, N.; Vergassola, C.; Pende, A.; Lotti, G. Simultaneous Measurement of Plasma Catecholamine (Norepinephrine, Epinephrine, and Dopamine) and Free N—Methyl Dopamine (Epinine) Levels, by HPLC with Electrochemical Detection. J. Liq. Chromatogr. 1990, 13, 2217–2228. [Google Scholar] [CrossRef]
- Hua, C.; Lee, H.K.; Hsieh, A.K. Determination of epinine in human urine by high-performance liquid chromatography coupled with electrochemical detection using carbon fiber microelectrodes. Electroanalysis 1994, 6, 1147–1149. [Google Scholar] [CrossRef]
- Hardee, G.E.; Lai, J.W. Determination of dobutamine in plasma by liquid chromatography with electrochemical detection. Anal. Lett. 1983, 16, 69–75. [Google Scholar] [CrossRef]
- Husseini, H.; Mitrovic, V.; Schlepper, M. Rapid and sensitive assay of dobutamine in plasma by high-performance liquid chromatography and electrochemical detection. J. Chromatogr. B Biomed. Appl. 1993, 620, 164–168. [Google Scholar] [CrossRef]
- Alberts, G.; Boomsma, F.; in’t Veld, A.M.; Schalekamp, M. Simultaneous determination of catecholamines and dobutamine in human plasma and urine by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Appl. 1992, 583, 236–240. [Google Scholar] [CrossRef]
- Szlag, V.M.; Rodriguez, R.S.; Jung, S.; Bourgeois, M.R.; Bryson, S.; Purchel, A.; Schatz, G.C.; Haynes, C.L.; Reineke, T.M. Optimizing linear polymer affinity agent properties for surface-enhanced Raman scattering detection of aflatoxin B1. Mol. Syst. Des. Eng. 2019, 4, 1019–1031. [Google Scholar] [CrossRef]
- El-Kommos, M.E. Spectrophotometric determination of dobutamine hydrochloride using 3-methylbenzothiazolin-2-one hydrazone. Analyst 1987, 112, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.S.; Chen, Y.H.; Liang, H.Y. Determination of dobutamine hydrochloride by enzymatic catalytic spectrofluorimetry. J. Lumin. 2014, 29, 92–95. [Google Scholar] [CrossRef]
- Li, H.-F.; Cai, Z.; Lin, J.-M. Separation of catecholamines by microchip electrophoresis with a simple integrated laser-induced fluorescence detector. Anal. Chim. Acta 2006, 565, 183–189. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.; Zhang, Z.; Aizawa, M. Flow injection chemiluminescence determination of catecholamines with electrogenerated hypochlorite. Anal. Chim. Acta 1998, 374, 105–110. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Sheikhshoaie, M.; Sheikhshoaie, I.; Ranjbar, M.; Alizadeh, J.; Maxakato, N.W.; Abbaspourrad, A. A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode. New J. Chem. 2019, 43, 2362–2367. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef] [PubMed]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–443. [Google Scholar] [CrossRef]
- Jamali, T.; Karimi-Maleh, H.; Khalilzadeh, M.A. A novel nanosensor based on Pt: Co nanoalloy ionic liquid carbon paste electrode for voltammetric determination of vitamin B9 in food samples. LWT Food Sci. Technol. 2014, 57, 679–685. [Google Scholar] [CrossRef]
- Negahban, S.; Fouladgar, M.; Amiri, G. Improve the performance of carbon paste electrodes for determination of dobutamine using MnZnFe2O4 nanoparticles and ionic liquid. J. Taiwan Inst. Chem. Eng. 2017, 78, 51–55. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, Y.M.; Ekram, H. Design strategy and preparation of a conductive layered electrochemical sensor for simultaneous determination of ascorbic acid, dobutamine, acetaminophen and amlodipine. Sens. Actuators B Chem. 2019, 297, 126648. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 132928. [Google Scholar] [CrossRef]
- Fatima, B.; Saeed, U.; Hussain, D.; Rafiq, H.S.; Majeed, S.; Manzoor, S.; Qadir, S.Y.; Ashiq, M.N.; Najam-ul-Haq, M. Facile Hydrothermal Synthesis of NiTe Nanorods for Non-Enzymatic Electrochemical Sensing of Whole Blood Hemoglobin in Pregnant Anemic Women. Anal. Chim. Acta 2021, 1189, 339204. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Jahani, P.M.; Sheikhshoaie, I.; Beitollahi, H.; Asl, M.S.; Jang, H.W.; Shokouhimehr, M. Recent developments in voltammetric and amperometric sensors for cysteine detection. RSC Adv. 2021, 11, 5411–5425. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Karimi-Maleh, H.; Khoshnama, Z.; Hassankhani, A.; Abbasghorbani, M. A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food Anal. Methods 2015, 8, 549–557. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. Synergistic electrocatalytic activity of In2O3@FMWCNTs nanocomposite for electrochemical quantification of dobutamine in clinical patient blood and in injection dosage form. Talanta 2020, 208, 120362. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Garkani-Nejad, F.; Tajik, S.; Beitollahi, H.; Pourbasheer, E.; Larijanii, B. Determination of salicylic acid by differential pulse voltammetry using ZnO/Al2O3 nanocomposite modified graphite screen printed electrode. Int. J. Electrochem. Sci. 2017, 12, 9972–9982. [Google Scholar] [CrossRef]
- Ling, Y.Y.; Huang, Q.A.; Feng, D.X.; Li, X.Z.; Wei, Y. Electrochemical oxidation of dobutamine on a magnesium oxide microflowers–nafion composite film modified glassy carbon electrode. Anal. Methods 2013, 5, 4580–4584. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Tahernejad-Javazmi, F.; Ensafi, A.A.; Moradi, R.; Mallakpour, S.; Beitollahi, H. A high sensitive biosensor based on FePt/CNTs nanocomposite/N-(4-hydroxyphenyl)-3,5-dinitrobenzamide modified carbon paste electrode for simultaneous determination of glutathione and piroxicam. Biosens. Bioelectron. 2014, 60, 1–7. [Google Scholar] [CrossRef]
- Muniraj, V.K.A.; Kamaja, C.K.; Shelke, M.V. RuO2· nH2O nanoparticles anchored on carbon nano-onions: An efficient electrode for solid state flexible electrochemical supercapacitor. ACS Sustain. Chem. Eng. 2016, 4, 2528–2534. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Yamauchi, Y.; Shokouhimehr, M. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A. 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Khalilzadeh, M.A. Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples. Food Anal. Methods 2013, 6, 1639–1647. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.; Nan, F.; Zhao, Y.; Wang, P.; He, F.; Wang, Y. Mesoporous CuCo2O4 rods modified glassy carbon electrode as a novel non-enzymatic amperometric electrochemical sensors with high-sensitive ascorbic acid recognition. J. Alloys Compd. 2021, 852, 157045. [Google Scholar] [CrossRef]
- Tahernejad-Javazmi, F.; Shabani-Nooshabadi, M.; Karimi-Maleh, H. Analysis of glutathione in the presence of acetaminophen and tyrosine via an amplified electrode with MgO/SWCNTs as a sensor in the hemolyzed erythrocyte. Talanta 2018, 176, 208–213. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: A docking/experimental investigation. J. Ind. Eng. Chem. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Gupta, V.K.; Karimi-Maleh, H.; Sadegh, R. Simultaneous determination of hydroxylamine, phenol and sulfite in water and waste water samples using a voltammetric nanosensor. Int. J. Electrochem. Sci. 2015, 10, 303–316. [Google Scholar]
- Garkani Nejad, F.; Beitollahi, H.; Tajik, S.; Jahani, S. La3+-doped Co3O4 nanoflowers modified graphite screen printed electrode for electrochemical sensing of vitamin B6. Anal. Bioanal. Chem. Res. 2019, 6, 69–79. [Google Scholar]
- Sahoo, S.; Sahoo, P.; Sharma, A.; Satpati, A. Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens. Actuators B Chem. 2020, 309, 127763. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhang, J.-L.; Liu, J.-M.; Xiong, Z.-H.; Chen, X. Selective and competitive adsorption of azo dyes on the metal–organic framework ZIF-67. Air Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Hu, J.; Stein, A.; Tang, B. Nanocomposites of zeolitic imidazolate frameworks on graphene oxide for pseudocapacitor applications. J. Appl. Electrochem. 2016, 46, 441–450. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, W.; Chen, Y.; Xu, C.; Liu, R.; Han, Z. Removal of boron from water by GO/ZIF-67 hybrid material adsorption. Environ. Sci. Pollut. Res. 2020, 27, 28396–28407. [Google Scholar] [CrossRef]
- Shahmirzaee, M.; Hemmati-Sarapardeh, A.; Husein, M.M.; Schaffie, M.; Ranjbar, M. Development of a powerful zeolitic imidazolate framework (ZIF-8)/carbon fiber nanocomposite for separation of hydrocarbons and crude oil from wastewater. Microporous Mesoporous Mater. 2020, 307, 110463. [Google Scholar] [CrossRef]
- Ethiraj, J.; Bonino, F.; Lamberti, C.; Bordiga, S. H2S interaction with HKUST-1 and ZIF-8 MOFs: A multitechnique study. Microporous Mesoporous Mater. 2015, 207, 90–94. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; Volume 2, pp. 580–632. [Google Scholar]
- Tavana, T.; Rezvani, A.R.; Karimi-Maleh, H. Pt-doped NiO nanoparticle-ionic liquid modified electrochemical sensor: A powerful approach for determination of epinine in the presence of phenylephrine as two blood pressure raising drugs. Electroanalysis 2020, 32, 1828–1833. [Google Scholar] [CrossRef]





| Epinine Current (µA) | Interference | Percentage change in the Current in the Presence of Interference |
|---|---|---|
| 5.5 | K+, Cl−, Ca2+, Na+, Mg2+, and NO2− | ±2% |
| 5.5 | Vitamin B9, vitamin B6, tryptophan, alanine, citric acid, and phenylalanine | 3% to 4% |
| 5.5 | Methyldopa, dopamine, levodopa, carbidopa, isoproterenol, and epinephrine | 90% to 100% |
| Sample | Spiked | Found | Recovery (%) | Relative Standard Deviation (RSD; %) | ||||
|---|---|---|---|---|---|---|---|---|
| Epinine | Dobutamine | Epinine | Dobutamine | Epinine | Dobutamine | Epinine | Dobutamine | |
| Dobutamine ampoule | 0 | 0 | - | 2.5 | - | - | - | 3.2 |
| 5.0 | 2.5 | 5.1 | 4.9 | 102.0 | 98.0 | 2.3 | 2.9 | |
| 10.0 | 5.0 | 9.9 | 7.7 | 99.0 | 102.7 | 3.5 | 1.9 | |
| 15.0 | 7.5 | 15.5 | 9.7 | 103.3 | 97.0 | 2.6 | 2.8 | |
| 20.0 | 10.0 | 19.5 | 12.7 | 97.5 | 101.6 | 1.8 | 2.4 | |
| Urine | 0 | 0 | - | - | - | - | - | - |
| 5.0 | 7.5 | 5.1 | 7.4 | 102.0 | 98.7 | 2.8 | 2.8 | |
| 7.5 | 12.5 | 7.3 | 12.6 | 97.3 | 100.8 | 3.5 | 1.9 | |
| 10.0 | 17.5 | 9.9 | 18.1 | 99.0 | 103.4 | 1.7 | 3.3 | |
| 12.5 | 22.5 | 12.6 | 22.2 | 100.8 | 98.7 | 2.7 | 2.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahsavari, M.; Mortazavi, M.; Tajik, S.; Sheikhshoaie, I.; Beitollahi, H. Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine. Micromachines 2022, 13, 88. https://doi.org/10.3390/mi13010088
Shahsavari M, Mortazavi M, Tajik S, Sheikhshoaie I, Beitollahi H. Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine. Micromachines. 2022; 13(1):88. https://doi.org/10.3390/mi13010088
Chicago/Turabian StyleShahsavari, Mahboobeh, Mojtaba Mortazavi, Somayeh Tajik, Iran Sheikhshoaie, and Hadi Beitollahi. 2022. "Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine" Micromachines 13, no. 1: 88. https://doi.org/10.3390/mi13010088
APA StyleShahsavari, M., Mortazavi, M., Tajik, S., Sheikhshoaie, I., & Beitollahi, H. (2022). Synthesis and Characterization of GO/ZIF-67 Nanocomposite: Investigation of Catalytic Activity for the Determination of Epinine in the Presence of Dobutamine. Micromachines, 13(1), 88. https://doi.org/10.3390/mi13010088







