Enrichment of SOX2-Positive Cells in BRAF V600E Mutated and Recurrent Ameloblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Specimens
2.2. Immunohistochemistry and Immunofluorescence of SOX2 and Ki67
2.3. Assessment
2.4. Cell Lines and Cultures
2.5. Cell Viability Assay
2.6. Knockdown of SOX2
2.7. Immunocytofluorescence Assay
2.8. PCR and Sanger Sequencing
2.9. Treatment of BRAF Inhibitors
2.10. RNA Purification, Reverse Transcription, and Quantitative Real-Time PCR (qRT-PCR)
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
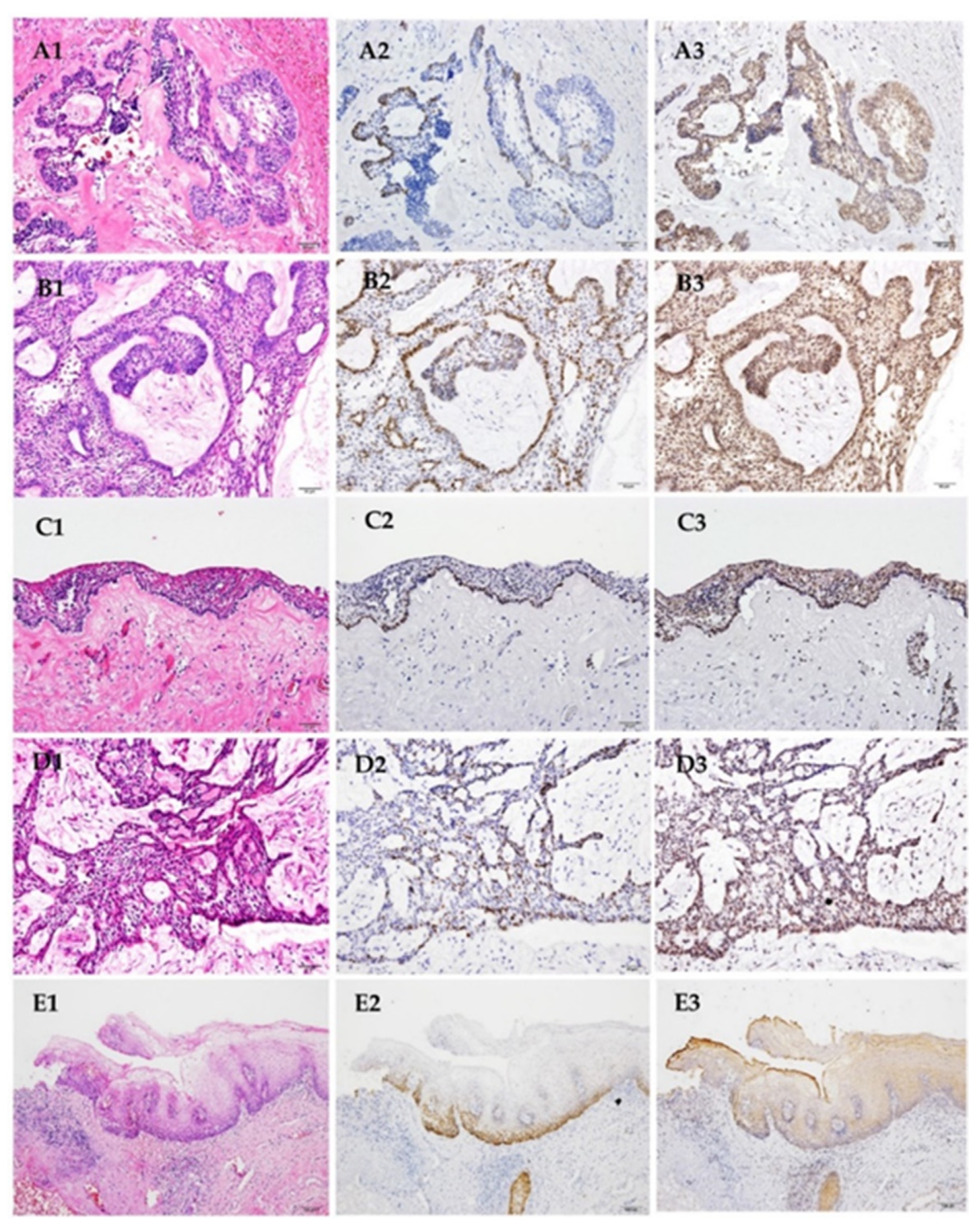
3.1. Verifying Specificity of Antibody for Detection of SOX2-Distinct Expression Patterns of SOX2 in Three Types of Ameloblastoma under Different Anti-SOX2 Antibodies
3.2. Remnants of Odontogenic Epithelium in Dental Follicle Containing SOX2+ Cells
3.3. Expression of SOX2 in Ameloblastoma
3.4. Expression of Ki-67 in Ameloblastoma
3.5. Cells Expressing SOX2+ or Ki-67+ Are Located in Different Populations, Suggesting SOX2+ Cells Are Most Likely Quiescent Stem Cells
3.6. Correlation of Expression Status of SOX2 and Ki-67 with Clinicopathological Parameters and Findings
3.6.1. Clinical Parameters—Recurrent Lesions Showed Higher SOX2 Positivity Comparing to Original Samples
3.6.2. Pathological Parameters and Findings
3.7. SOX2 Knockdown in Ameloblastoma Cell Lines Reduced Cellular Viability
3.8. Increased SOX2-Positive Cells in BRAF(V600E) Mutated Ameloblastomas
3.9. Ameloblastoma Resistant Clones Show Upregulation of SOX2 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 215–218. [Google Scholar]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Oral and Maxillofacial Pathology, 4th ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 653–661. [Google Scholar]
- Kurppa, K.J.; Catón, J.; Morgan, P.R.; Ristimäki, A.; Ruhin, B.; Kellokoski, J.; Elenius, K.; Heikinheimo, K. High frequency of BRAF V600E mutations in ameloblastoma. J. Pathol. 2014, 232, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.A.; Rolland, D.; McHugh, J.B.; Weigelin, H.C.; Zhao, L.; Lim, M.S.; Elenitoba-Johnson, K.S.; Betz, B.L. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin. Cancer Res. 2014, 20, 5517–5526. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, R.T.; McClary, A.C.; Myers, B.R.; Biscocho, J.; Neahring, L.; Kwei, K.A.; Qu, K.; Gong, X.; Ng, T.; Jones, C.D.; et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat. Genet. 2014, 46, 722–725. [Google Scholar] [CrossRef] [Green Version]
- Hombach-Klonisch, S.; Panigrahi, S.; Rashedi, I.; Seifert, A.; Alberti, E.; Pocar, P.; Kurpisz, M.; Schulze-Osthoff, K.; Mackiewicz, A.; Los, M. Adult stem cells and their trans-differentiation potential—Perspectives and therapeutic applications. J. Mol. Med. 2008, 86, 1301–1314. [Google Scholar] [CrossRef] [Green Version]
- Klonisch, T.; Wiechec, E.; Hombach-Klonisch, S.; Ande, S.R.; Wesselborg, S.; Schulze-Osthoff, K.; Los, M. Cancer stem cell markers in common cancers—Therapeutic implications. Trends Mol. Med. 2008, 14, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgard, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299. [Google Scholar] [CrossRef]
- Suomalainen, M.; Thesleff, I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev. Dyn. 2010, 239, 364–372. [Google Scholar]
- Juuri, E.; Saito, K.; Ahtiainen, L.; Seidel, K.; Tummers, M.; Hochedlinger, K.; Klein, O.D.; Thesleff, I.; Michon, F. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 2012, 23, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014, 511, 246–250. [Google Scholar] [CrossRef]
- Fang, W.T.; Fan, C.C.; Li, S.M.; Jang, T.H.; Lin, H.P.; Shih, N.Y.; Chen, C.H.; Wang, T.Y.; Huang, S.F.; Lee, A.Y.; et al. Downregulation of a putative tumor suppressor BMP4 by SOX2 promotes growth of lung squamous cell carcinoma. Int. J. Cancer 2014, 135, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Li, X.; Xu, Y.; Zhang, S.; Mou, W.; Liu, Y.; Liu, Y.; Lv, D.; Liu, C.H.; Tan, X.; et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J. Mol. Cell Biol. 2011, 3, 230–238. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Lu, D.; Xu, Y.; Mou, W.; Wang, L.; Chen, Y.; Liu, Y.; Li, X.; Li, L.Y.; et al. SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells. Carcinogenesis 2014, 35, 613–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girouard, S.D.; Laga, A.C.; Mihm, M.C.; Scolyer, R.A.; Thompson, J.F.; Zhan, Q.; Widlund, H.R.; Lee, C.W.; Murphy, G.F. SOX2 contributes to melanoma cell invasion. Lab. Investig. 2012, 92, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.M.; Diez-Valle, R.; Manterola, L.; Rubio, A.; Liu, D.; Cortes-Santiago, N.; Urquiza, L.; Jauregi, P.; Lopez de Munain, A.; Sampron, N.; et al. Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PLoS ONE 2011, 6, e26740. [Google Scholar] [CrossRef]
- Han, X.; Fang, X.; Lou, X.; Hua, D.; Ding, W.; Foltz, G.; Hood, L.; Yuan, Y.; Lin, B. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS ONE 2012, 7, e41335. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Trevino, J.; Bora-Singhal, N.; Coppola, D.; Haura, E.; Altiok, S.; Chellappan, S.P. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol. Cancer 2012, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/beta-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, L.; Li, Y.; Kang, X.; Zhang, S.; Liu, Y. Sox2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med. Oncol. 2013, 30, 503. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jia, X.; Wang, S.; Li, Y.; Zhao, P.; Cai, D.; Zhou, Z.; Wang, J.; Luo, Y.; Dong, M. SOX2 oncogenes amplified and operate to activate AKT signaling in gastric cancer and predict immunotherapy responsiveness. J. Cancer Res. Clin. Oncol. 2014, 140, 1117–1124. [Google Scholar] [CrossRef]
- Schröck, A.; Göke, F.; Wagner, P.; Bode, M.; Franzen, A.; Braun, M.; Huss, S.; Agaimy, A.; Ihrler, S.; Menon, R.; et al. Sex determining region Y-box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PLoS ONE 2013, 8, e59201. [Google Scholar] [CrossRef]
- Neumann, J.; Bahr, F.; Horst, D.; Kriegl, L.; Engel, J.; Luque, R.M.; Gerhard, M.; Kirchner, T.; Jung, A. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer 2011, 11, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; He, W.; Lu, C.; Wang, Z.; Wang, J.; Giercksky, K.E.; Nesland, J.M.; Suo, Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009, 29, 1233–1241. [Google Scholar]
- Lundberg, I.V.; Löfgren Burström, A.; Edin, S.; Eklöf, V.; Öberg, Å.; Stenling, R.; Palmqvist, R.; Wikberg, M.L. SOX2 expression is regulated by BRAF and contributes to poor patient prognosis in colorectal cancer. PLoS ONE 2014, 9, e101957. [Google Scholar] [CrossRef] [Green Version]
- Juuri, E.; Isaksson, S.; Jussila, M.; Heikinheimo, K.; Thesleff, I. Expression of the stem cell marker, SOX2, in ameloblastoma and dental epithelium. Eur. J. Oral Sci. 2013, 121, 509–516. [Google Scholar] [CrossRef]
- Lei, Y.; Jaradat, J.M.; Owosho, A.; Adebiyi, K.E.; Lybrand, K.S.; Neville, B.W.; Muller, S.; Bilodeau, E.A. Evaluation of SOX2 as a potential marker for ameloblastic carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 608–616.e601. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.P.; Liu, I.J.; Chiang, C.P.; Wu, H.C. Astrocyte elevated gene-1 is associated with metastasis in head and neck squamous cell carcinoma through p65 phosphorylation and upregulation of MMP1. Mol. Cancer 2013, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Liu, I.J.; Chiu, C.Y.; Chen, Y.C.; Wu, H.C. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J. Biol. Chem. 2011, 286, 9726–9736. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H.; Chang, J.Y.F. Correlation of SOX2 expression with biologic behavior in odontogenic neoplasms with ameloblastic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e171. [Google Scholar] [CrossRef]
- Hu, J.C.; Chun, Y.H.; Al Hazzazzi, T.; Simmer, J.P. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs 2007, 186, 78–85. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Sandra, F.; Mitsuyasu, T.; Nakamura, N.; Shiratsuchi, Y.; Ohishi, M. Immunohistochemical evaluation of PCNA and Ki-67 in ameloblastoma. Oral Oncol. 2001, 37, 193–198. [Google Scholar] [CrossRef]
- Meer, S.; Galpin, J.S.; Altini, M.; Coleman, H.; Ali, H. Proliferating cell nuclear antigen and Ki67 immunoreactivity in ameloblastomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 213–221. [Google Scholar] [CrossRef]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Lopez-Corella, E.; Almeida, O.P.; Carrasco-Daza, D.; Garcia-Vazquez, F.; Farfan-Morales, J.E.; Irigoyen-Camacho, M.E.; Damian-Matsumura, P. Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol. 2008, 44, 805–811. [Google Scholar] [CrossRef]
- Vanner, R.J.; Remke, M.; Gallo, M.; Selvadurai, H.J.; Coutinho, F.; Lee, L.; Kushida, M.; Head, R.; Morrissy, S.; Zhu, X.; et al. Quiescent sox2+ cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 2014, 26, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.L.; Chen, W.S.; Li, J.; Lind, A.C.; Lu, D. Diagnostic utility of neural stem and progenitor cell markers nestin and SOX2 in distinguishing nodal melanocytic nevi from metastatic melanomas. Mod. Pathol. 2013, 26, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Hu, F.W.; Yu, C.H.; Yu, C.C. Sox2 expression involvement in the oncogenicity and radiochemoresistance of oral cancer stem cells. Oral Oncol. 2015, 51, 31–39. [Google Scholar] [CrossRef]
- Liu, K.; Xie, F.; Zhao, T.; Zhang, R.; Gao, A.; Chen, Y.; Li, H.; Zhang, S.; Xiao, Z.; Li, J.; et al. Targeting SOX2 protein with peptide aptamers for therapeutic gains against esophageal squamous cell carcinoma. Mol. Ther. 2020, 28, 901–913. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Dabrafenib Plus Trametinib for Adjuvant Treatment of Melanoma with BRAF V600E or V600K Mutations. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-adjuvant-treatment-melanoma-braf-v600e-or-v600k-mutations (accessed on 18 June 2021).
- Tan, S.; Pollack, J.R.; Kaplan, M.J.; Colevas, A.D.; West, R.B. BRAF inhibitor treatment of primary BRAF-mutant ameloblastoma with pathologic assessment of response. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e5–e7. [Google Scholar] [CrossRef]
- Kaye, F.J.; Ivey, A.M.; Drane, W.E.; Mendenhall, W.M.; Allan, R.W. Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J. Natl. Cancer Inst. 2015, 107, 378. [Google Scholar] [CrossRef] [Green Version]
- Faden, D.L.; Algazi, A. Durable treatment of ameloblastoma with single agent BRAFi Re: Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J. Natl. Cancer Inst. 2017, 109, djw190. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.S.; Girardi, D.M.; Bernardes, J.P.G.; Fonseca, F.P.; Fregnani, E.R. Clinical benefit and radiological response with BRAF inhibitor in a patient with recurrent ameloblastoma harboring V600E mutation. BMC Cancer 2018, 18, 887. [Google Scholar] [CrossRef]
- Proietti, I.; Skroza, N.; Bernardini, N.; Tolino, E.; Balduzzi, V.; Marchesiello, A.; Michelini, S.; Volpe, S.; Mambrin, A.; Mangino, G.; et al. Mechanisms of acquired BRAF inhibitor resistance in melanoma: A systematic review. Cancers 2020, 12, 2801. [Google Scholar] [CrossRef]
- Mao, M.; Tian, F.; Mariadason, J.M.; Tsao, C.C.; Lemos, R., Jr.; Dayyani, F.; Gopal, Y.N.; Jiang, Z.Q.; Wistuba, I.I.; Tang, X.M.; et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin. Cancer Res. 2013, 19, 657–667. [Google Scholar] [CrossRef] [Green Version]








| Follicular Type (n = 27) | Plexiform Type (n = 22) | Unicystic Type (n = 25) | |
|---|---|---|---|
| Age (year) | |||
| <20 | 1 | 8 | 9 |
| 21 to 40 | 9 | 8 | 12 |
| ≥41 | 17 | 6 | 4 |
| Gender | |||
| Men | 15 | 18 | 12 |
| Women | 12 | 4 | 13 |
| Location | |||
| Maxilla | 5 | 0 | 0 |
| Mandible | 22 | 22 | 25 |
| Bone perforation | |||
| Present | 16 | 14 | 13 |
| Absent | 2 | 6 | 4 |
| Root resorption | |||
| Present | 15 | 13 | 11 |
| Absent | 7 | 7 | 11 |
| Size of lesion a | |||
| <100,000 | 7 | 9 | 15 |
| 100,001 to 200,000 | 10 | 5 | 8 |
| ≥200,001 | 5 | 6 | 1 |
| Case Number | Labeling Indices of SOX2 Immunostain (%) |
|---|---|
| DF-1 | 33.8 |
| DF-2 | 47.3 |
| DF-3 | 45.7 |
| DF-4 | 47.8 |
| DF-5 | 59.2 |
| DF-6 | 34.1 |
| Mean ± S.D. (%): 44.7 ± 9.6 Median (%): 46.5 |
| Follicular Type (n = 27) | Plexiform Type (n = 22) | Unicystic Type (n = 25) | |
|---|---|---|---|
| Ratio of high columnar cells in SOX2+ cells | |||
| <2 | 20 | 21 | 20 |
| ≥2 | 7 | 1 | 5 |
| Labeling index of SOX2 (%) | |||
| <10 | 17 | 5 | 8 |
| 11 to 20 | 3 | 4 | 7 |
| >20 | 7 | 13 | 10 |
| Labeling index of Ki-67 (%) | |||
| <3 | 21 | 11 | 14 |
| 3 to 6 | 3 | 6 | 7 |
| >6 | 3 | 5 | 4 |
| Histologic Type | Mean ± S.D. (%) | Median (%) | Comparing with | pa |
|---|---|---|---|---|
| Follicular type | 17.2 ± 21.9 | 6.2 | Plexiform type | 0.031 |
| Unicystic type | 0.107 | |||
| Plexiform type | 28.8 ± 22.1 | 23.3 | Follicular type | 0.031 |
| Unicystic type | 0.216 | |||
| Unicystic type | 20.3 ± 16.5 | 14.2 | Follicular type | 0.107 |
| Plexiform type | 0.216 |
| Histologic Type | Mean ± S.D. (%) | Median (%) | Comparing with | pa |
|---|---|---|---|---|
| Follicular type | 2.5 ± 2.0 | 2.0 | Plexiform type | 0.048 |
| Unicystic type | 0.674 | |||
| Plexiform type | 4.1 ± 3.3 | 3.0 | Follicular type | 0.048 |
| Unicystic type | 0.179 | |||
| Unicystic type | 3.3 ± 3.1 | 1.8 | Follicular type | 0.674 |
| Plexiform type | 0.179 |
| Ameloblastoma | BRAF(V600E) Wild Type | BRAF(V600E) Mutant | ||||
|---|---|---|---|---|---|---|
| Subtype | Follicular | Plexiform (n = 2) | Unicystic (n = 5) | Follicular (n = 18) | Plexiform (n = 14) | Unicystic (n = 16) |
| SOX2-positive cells | - | 7.25% ± 0.03% | 6.3% ± 0.02% | 21.74% ± 0.22% | 35.05% ± 0.2% | 18.87% ± 0.15% |
| Average | 6.57% ± 0.02% | 24.54% ± 0.21% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-H.; Lu, P.-H.; Wang, Y.-P.; Chang, J.Y.F. Enrichment of SOX2-Positive Cells in BRAF V600E Mutated and Recurrent Ameloblastoma. J. Pers. Med. 2022, 12, 77. https://doi.org/10.3390/jpm12010077
Tseng C-H, Lu P-H, Wang Y-P, Chang JYF. Enrichment of SOX2-Positive Cells in BRAF V600E Mutated and Recurrent Ameloblastoma. Journal of Personalized Medicine. 2022; 12(1):77. https://doi.org/10.3390/jpm12010077
Chicago/Turabian StyleTseng, Chih-Huang, Pei-Hsuan Lu, Yi-Ping Wang, and Julia Yu Fong Chang. 2022. "Enrichment of SOX2-Positive Cells in BRAF V600E Mutated and Recurrent Ameloblastoma" Journal of Personalized Medicine 12, no. 1: 77. https://doi.org/10.3390/jpm12010077
APA StyleTseng, C.-H., Lu, P.-H., Wang, Y.-P., & Chang, J. Y. F. (2022). Enrichment of SOX2-Positive Cells in BRAF V600E Mutated and Recurrent Ameloblastoma. Journal of Personalized Medicine, 12(1), 77. https://doi.org/10.3390/jpm12010077






