Petrography and Geochemistry of the Intrusive Rocks at the Diorite-Hosted Regnault Au Mineralization
Abstract
:1. Introduction
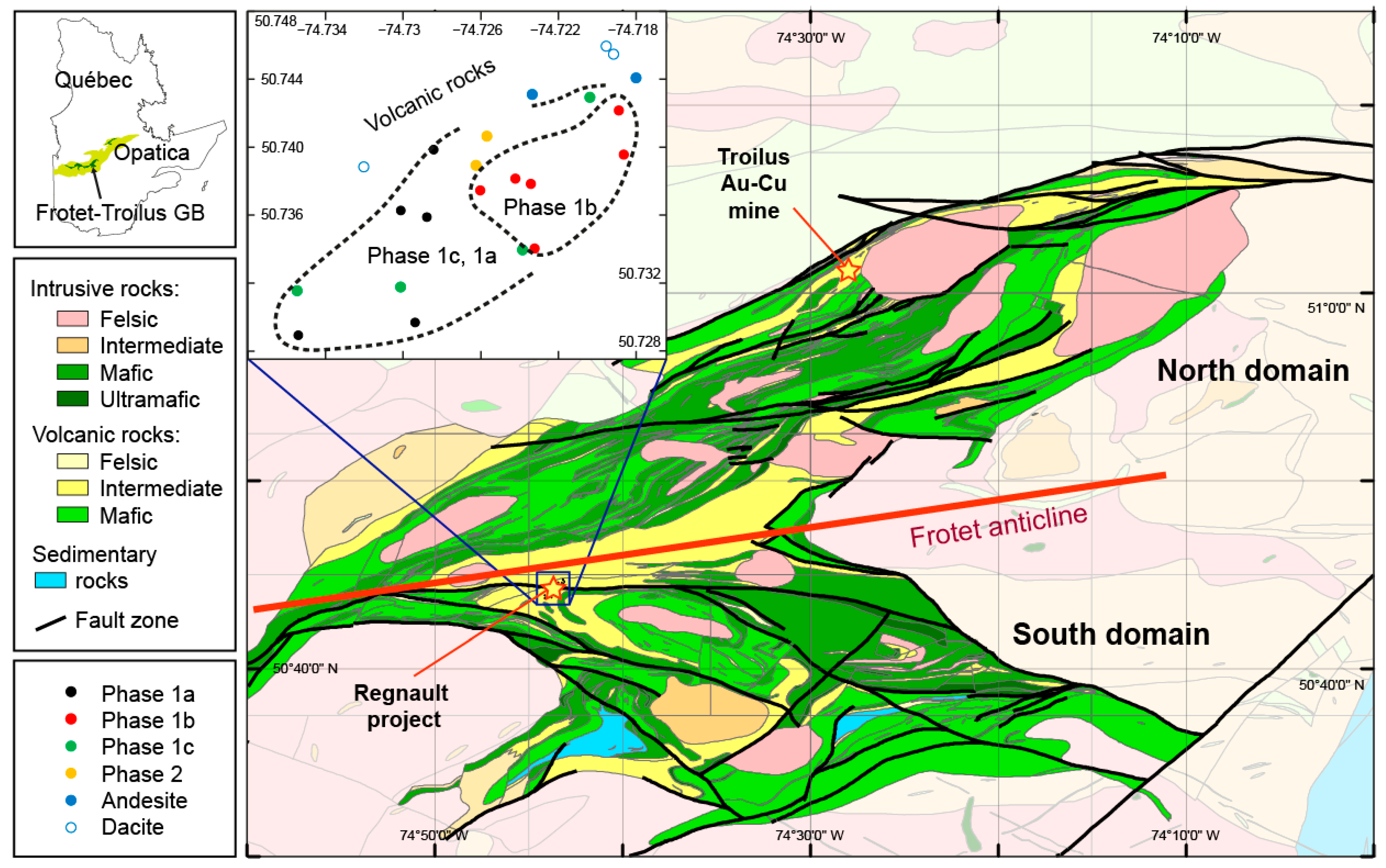
2. Geological Setting
2.1. Regional Geology
2.2. Regnault Project
3. Methodology
3.1. Whole-Rock Chemsitry
3.2. Petrography and Mineral Chemistry
4. Results
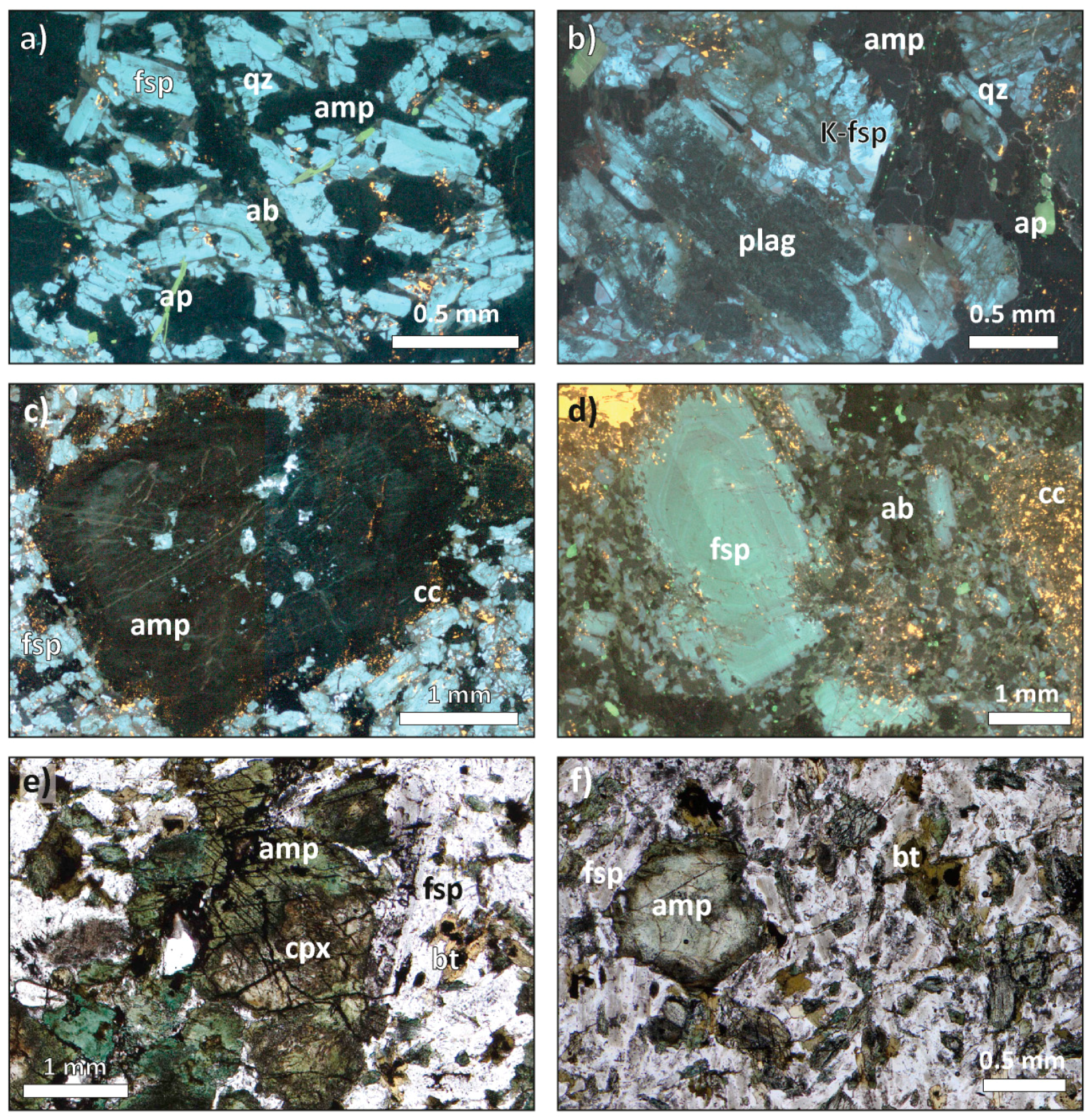
4.1. Petrography and Texture of the Main Lithologies
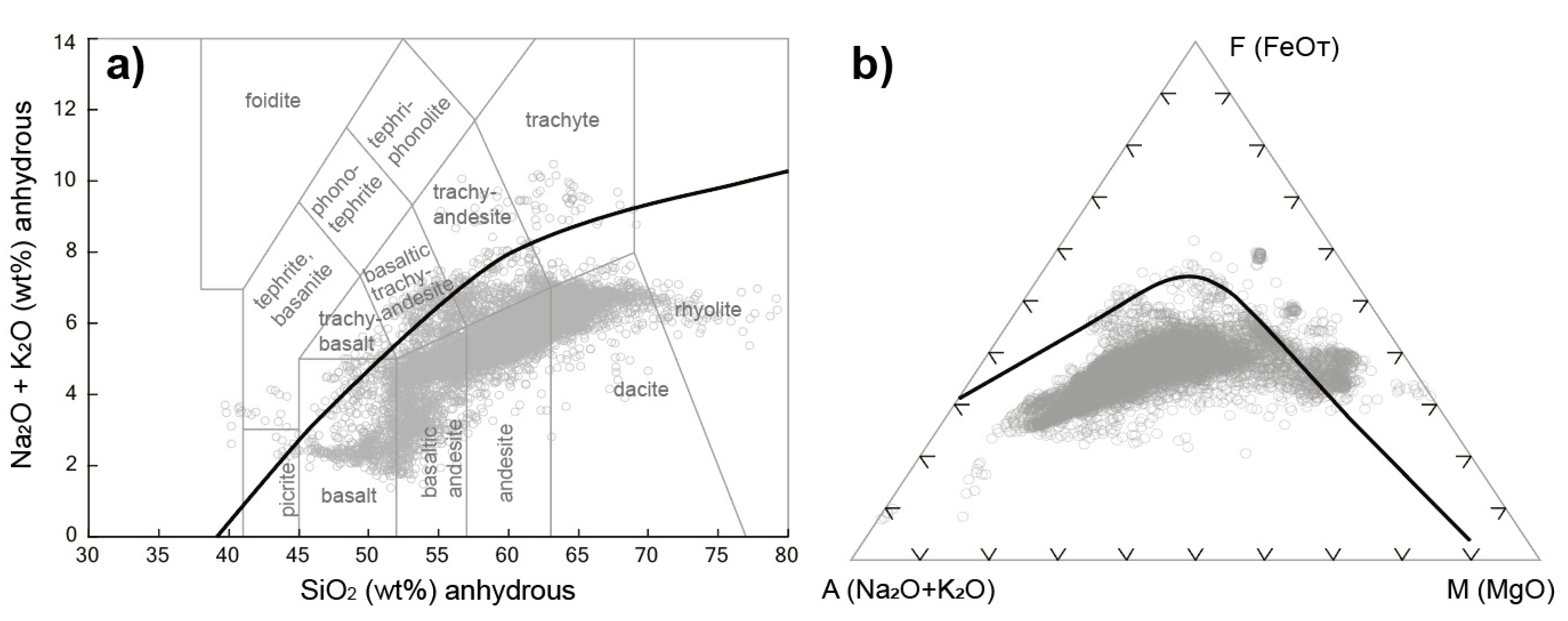
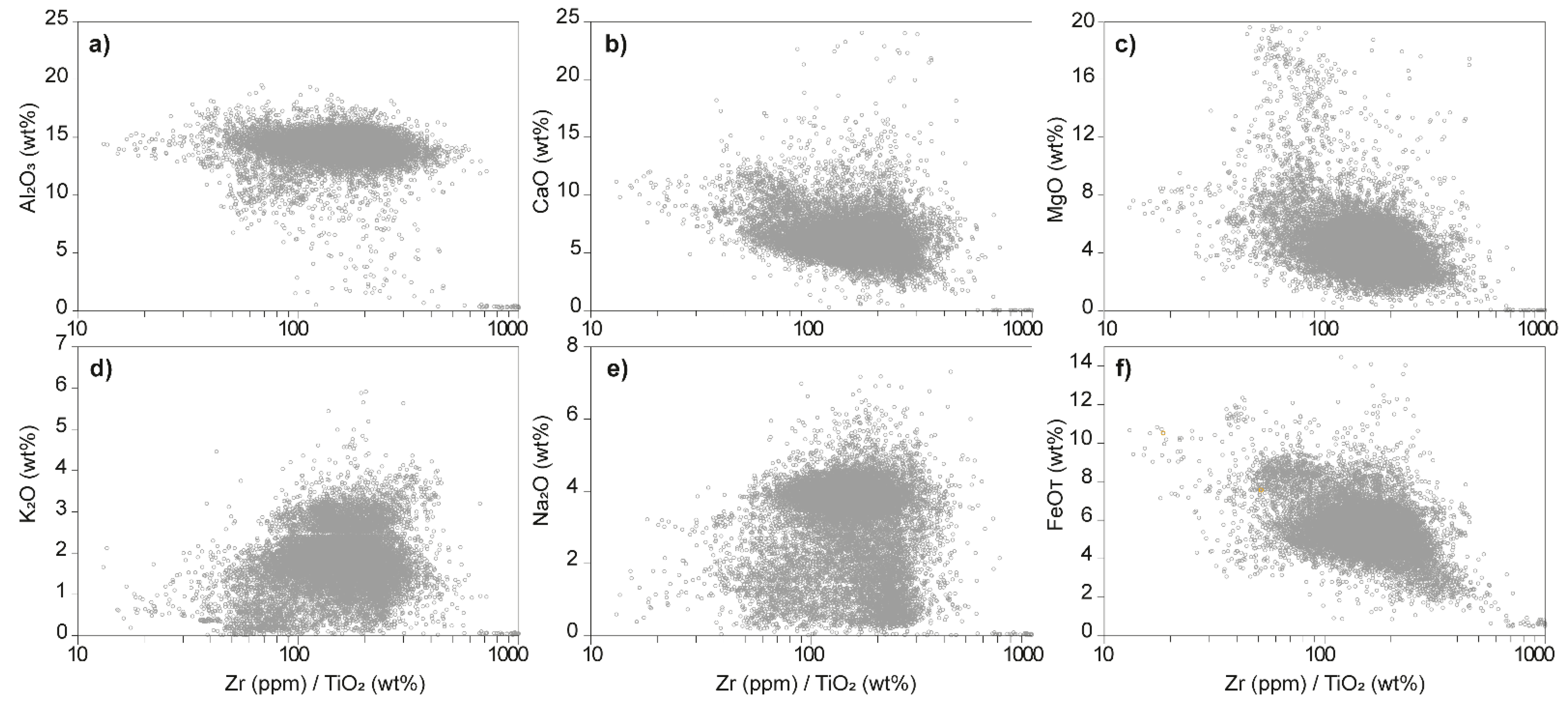
4.2. Whole-Rock Chemistry
4.3. Texture of the Main Minerals and Accessory Phases
4.4. Mineral Chemistry
4.5. Amphibole Chemistry as Determined by Laser Ablation
5. Discussion
5.1. Characteristics of the Granodiorite-Diorite-Gabbro Intrusive Complex of Regnault
5.2. Physical-Chemical Parameters Deduced from Amphibole Chemistry
5.3. Hydrothermal Alteration
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontaine, A.; Dubé, B.; Malo, M.; McNicoll, V.J.; Brisson, T.; Doucet, D.; Goutier, J. Geology of the metamorphosed Roberto gold deposit (Éléonore Mine), James Bay region, Quebec: Diversity of mineralization styles in a polyphase tectonometamorphic setting. In Targeted Geoscience Initiative 4: Contributions to the Understanding of Precambrian Lode Gold Deposits and Implications for Exploration; Dubé, B., Mercier-Langevin, P., Eds.; Geological Survey of Canada Open File 7852; Geological Survey of Canada: Québec, QC, Canada, 2015; pp. 209–227. [Google Scholar]
- Ravenelle, J.-F.; Dubé, B.; Malo, M.; McNicoll, V.; Nadeau, I.; Simoneau, J. Insights on the Geology of the World-Class Roberto Gold Deposit, Éléonore Property, James Bay Area, Quebec; Current Research 2010-1; Geological Survey of Canada: Québec, QC, Canada, 2010; ISBN 1100145885. [Google Scholar]
- Fontaine, A.; Dubé, B.; Malo, M.; Turcotte, J.; Doucet, D. Geology of the Cheechoo Gold Property, Eeyou Istchee Baie-James, Superior Province, Northern Quebec; Geological Survey of Canada Open File 8403; Geological Survey of Canada: Québec, QC, Canada, 2018. [Google Scholar]
- Diniz, T.B. An Overview of the Troilus Au-Cu Deposit, Frotet-Evans Greenstone Belt, Opatica Subprovince, Quebec. Unpublished Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2019. [Google Scholar]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Mathieu, L. Intrusion-associated gold systems and multistage metallogenic. Miner. Depos. 2021, 11, 261. [Google Scholar]
- Katz, L.R.; Kontak, D.J.; Dubé, B.; McNicoll, V.; Creaser, R.; Petrus, J.A. An archean porphyry-type gold deposit: The côté gold Au (-Cu) deposit, swayze greenstone belt, superior province, Ontario, Canada. Econ. Geol. 2020, 116, 47–89. [Google Scholar] [CrossRef]
- Katz, L.R.; Kontak, D.J.; Dubé, B.; Mcnicoll, V. The geology, petrology, and geochronology of the Archean côté gold large-tonnage, low-grade intrusion-related Au(–Cu) deposit, Swayze greenstone belt, Ontario, Canada. Can. J. Earth Sci. 2017, 54, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, L.; Racicot, D. Petrogenetic study of the multiphase chibougamau pluton: Archaean magmas associated with cu-au magmato-hydrothermal systems. Minerals 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Pilote, P.; Guha, J. Partie B—Métallogénie de l’extrémité est de la sous-province de l’Abitibi. In Le Camp Minier de Chibougamau et le Parautochtone Grenvillien: Métallogénie, Métamorphisme et Aspects Structuraux; Pilote, P., Ed.; Geological Association of Canada Field Trip Guidebook B1: Québec, QC, Canada, 2006; pp. 29–46. [Google Scholar]
- Pilote, P.; Dion, C.; Joanisse, A.; David, J.; Machado, N.; Kirkham, R.V.; Robert, F. Géochronologie des minéralisations d’affiliation magmatique de l’Abitibi, secteurs Chibougamau et de Troilus-Frotet: Implications géotectoniques. In Programme et Résumés, Séminaire D’information Sur la Recherche Géologique; MRN Report, DV-97-03; Ministère des Ressources Naturelles: Québec, QC, Canada, 1997; p. 47. [Google Scholar]
- Goodman, S.; Williams-Jones, A.E.; Carles, P. Structural controls on the Archean Troilus gold-copper deposit, Quebec, Canada. Econ. Geol. 2005, 100, 577–582. [Google Scholar] [CrossRef]
- David, J.; Parent, M. Géochronologie U-Pb du Projet Moyen-Nord; MRNF Document GM 59903; Ministère des Ressources Naturelles et de la Faune: Québec, QC, Canada, 1997. [Google Scholar]
- El Rassi, D. Technical Report on the Douay Gold Project, Northwestern Québec, Canada; Technical Report NI 43-101; Maple Gold Mines Ltd.: Vancouver, BC, Canada, 2019. [Google Scholar]
- Gosselin, C. Synthèse Géologique de la Région de Frotet-Troïlus. Ministère des Ressources Naturelles, Québec; MERN Report ET 96-02; Ministère de l’Énergie et des Ressources Naturelles: Québec, QC, Canada, 1996. [Google Scholar]
- Davis, W.J.; Machado, N.; Gariépy, C.; Sawyer, E.W.; Benn, K. U-Pb geochronology of the Opatica tonalite-gneiss belt and its relationship to the Abitibi greenstone belt, superior province, Quebec. Can. J. Earth Sci. 1995, 32, 113–127. [Google Scholar] [CrossRef]
- Davis, W.J.; Gariepy, C.; Sawyer, E.W. Pre-2.8 Ga crust in the Opatica gneiss belt: A potential source of detrital zircons in the Abitibi and Pontiac subprovinces, superior province, Canada. Geology 1994, 22, 1111–1114. [Google Scholar] [CrossRef]
- Boily, M.; Dion, C. Geochemistry of boninite-type volcanic rocks in the Frotet-Evans greenstone belt, Opatica subprovince, Quebec: Implications for the evolution of Archaean greenstone belts. Precambr. Res. 2002, 115, 349–371. [Google Scholar] [CrossRef]
- Laurent, O.; Martin, H.; Moyen, J.-F.; Doucelance, R. The diversity and evolution of late-Archean granitoids: Evidence for the onset of “modern-style” plate tectonics between 3.0 and 2.5 Ga. Lithos 2014, 205, 208–235. [Google Scholar] [CrossRef]
- Benn, K.; Sawyer, E.W.; Bouchez, J.L. Orogen parallel and transverse shearing in the Opatica Belt, Quebec: Implications for the structure of the Abitibi Subprovince. Can. J. Earth Sci. 1992, 29, 2429–2444. [Google Scholar] [CrossRef]
- Sawyer, E.W.; Benn, K. Structure of the high-grade Opatica Belt and adjacent low-grade Abitibi Subprovince, Canada: An Archaean mountain front. J. Struct. Geol. 1993, 15, 1443–1458. [Google Scholar] [CrossRef]
- Simard, A. Stratigraphie et Volcanisme Dans la Partie Orientale de la Bande Volcano-Sédimentaire Archéenne Frotet-Evans; MERN Report MB 87-17; Ministère de l’Énergie et des Ressources Naturelles: Québec, QC, Canada, 1987. [Google Scholar]
- Magnan, M. La Zone 87 du Gisement d’or et De Cuivre du Lac Troilus: Pétrographie et Géochimie; Université du Québec à Chicoutimi: Saguenay, QC, Canada, 1993; ISBN 1412305314. [Google Scholar]
- Carles, P. Constraints on the Genesis of the Archean Troilus Gold-Copper Deposit, Quebec: Montreal. Unpublished Master’s Thesis, McGill University, Montréal, QC, Canada, 2000. [Google Scholar]
- Larouche, J. Caractérisation de la Minéralisation de la Fosse J4 à la Mine Troilus, Chibougamau. Unpublished Master’s Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2005. [Google Scholar]
- Hawkins, T.; Charbonneau, R. NI 43-101 Technical Report for the Frotet Gold Project; NI 43-101 Report; Northway Resources and Kenorland Minerals Ltd.: Vancouvert, BC, Canada, 2020. [Google Scholar]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A. Determination of reference values for NIST SRM 610-617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Jochum, K.P.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical characterisation of the USGS reference glasses GSA 1G, GSC 1G, GSD 1G, GSE 1G, BCR 2G, BHVO 2G and BIR 1G using EPMA, ID TIMS, ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 2005, 29, 285–302. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Trépanier, S.; Mathieu, L.; Daigneault, R.; Faure, S. Precursors predicted by artificial neural networks for mass balance calculations: Quantifying hydrothermal alteration in volcanic rocks. Comput. Geosci. 2016, 89, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.A. Geochemical fingerprinting of oceanic basalts with applications to ophiolite classification and the search for Archean oceanic crust. Lithos 2008, 100, 14–48. [Google Scholar] [CrossRef]
- Hofmann, A.W. Chemical differentiation of the Earth: The relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 1988, 90, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Henry, D.J.; Guidotti, C.V.; Thomson, J.A. The Ti-saturation surface for low-to-medium pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms. Am. Mineral. 2005, 90, 316–328. [Google Scholar] [CrossRef]
- Rezaei, M.; Zarasvandi, A. Titanium in biotite thermometry in porphyry copper systems: Challenges to application of the thermometer. Resour. Geol. 2020, 70, 157–168. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Ima report: Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Ridolfi, F.; Renzulli, A.; Puerini, M. Stability and chemical equilibrium of amphibole in calc-alkaline magmas: An overview, new thermobarometric formulations and application to subduction-related volcanoes. Contrib. Mineral. Petrol. 2010, 160, 45–66. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer Science & Business Media: Dordrecht, The Netherlands, 2009; ISBN 140208613X. [Google Scholar]
- Richards, J.P.; Mumin, A.H. Magmatic-hydrothermal processes within an evolving Earth: Iron oxide-copper-gold and porphyry Cu ± Mo ± Au deposits. Geology 2013, 41, 767–770. [Google Scholar] [CrossRef]
- Audétat, A.; Simon, A.C. Magmatic controls on porphyry copper genesis. In Geology and Genesis of Major Copper Deposits and Districts of the World—A Tribute to Richard H. Sillitoe; Society of Economic Geology: Littleton, CO, USA, 2020; Volume 16, pp. 553–572. ISBN 9781629490410. [Google Scholar]
- Burnham, C.W. Magmas and hydrothermal fluids. In Geochemistry of Hydrothermal Ore Deposits; Barnes, L.H., Ed.; John Wiley & Sons: New York, NY, USA, 1979; pp. 71–136. [Google Scholar]
- Nachit, H.; Ibhi, A.; Ohoud, M.B. Discrimination between primary magmatic biotites, reequilibrated biotites and neoformed biotites. Comptes Rendus Geosci. 2005, 337, 1415–1420. [Google Scholar] [CrossRef]
- Mathieu, L.; Crépon, A.; Kontak, D.J. Tonalite-dominated magmatism in the abitibi subprovince, canada, and significance for Cu-Au magmatic-hydrothermal systems. Minerals 2020, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Chiaradia, M. Copper enrichment in arc magmas controlled by overriding plate thickness. Nat. Geosci. 2014, 7, 43–46. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Poli, S. Magmatic epidote. Rev. Mineral. Geochem. 2004, 56, 399–430. [Google Scholar] [CrossRef]
- Smith, D.J. Clinopyroxene precursors to amphibole sponge in arc crust. Nat. Commun. 2014, 5, 4329. [Google Scholar] [CrossRef] [Green Version]
- Blake, S. Volatile oversaturation during the evolution of silicic magma chambers as an eruption trigger. J. Geophys. Res. Solid Earth 1984, 89, 8237–8244. [Google Scholar] [CrossRef]
- Botcharnikov, R.E.; Linnen, R.L.; Wilke, M.; Holtz, F.; Jugo, P.J.; Berndt, J. High gold concentrations in sulphide-bearing magma under oxidizing conditions. Nat. Geosci. 2011, 4, 112. [Google Scholar] [CrossRef]
- Dilles, J.H. Petrology of the Yerington Batholith, Nevada; evidence for evolution of porphyry copper ore fluids. Econ. Geol. 1987, 82, 1750–1789. [Google Scholar] [CrossRef]
- Spear, F.S. Metamorphic Phase Equilibria and Pressure-Temperature Time Paths. Mineralogical Society of America, Monograph; Monograph series; Mineralogical Society of America: Washington, DC, USA, 1993; Volume 1. [Google Scholar]
- Moyen, J.-F. Archean granitoids: Classification, petrology, geochemistry and origin. Geol. Soc. Lond. Spec. Publ. 2019, 489, 15–49. [Google Scholar] [CrossRef]

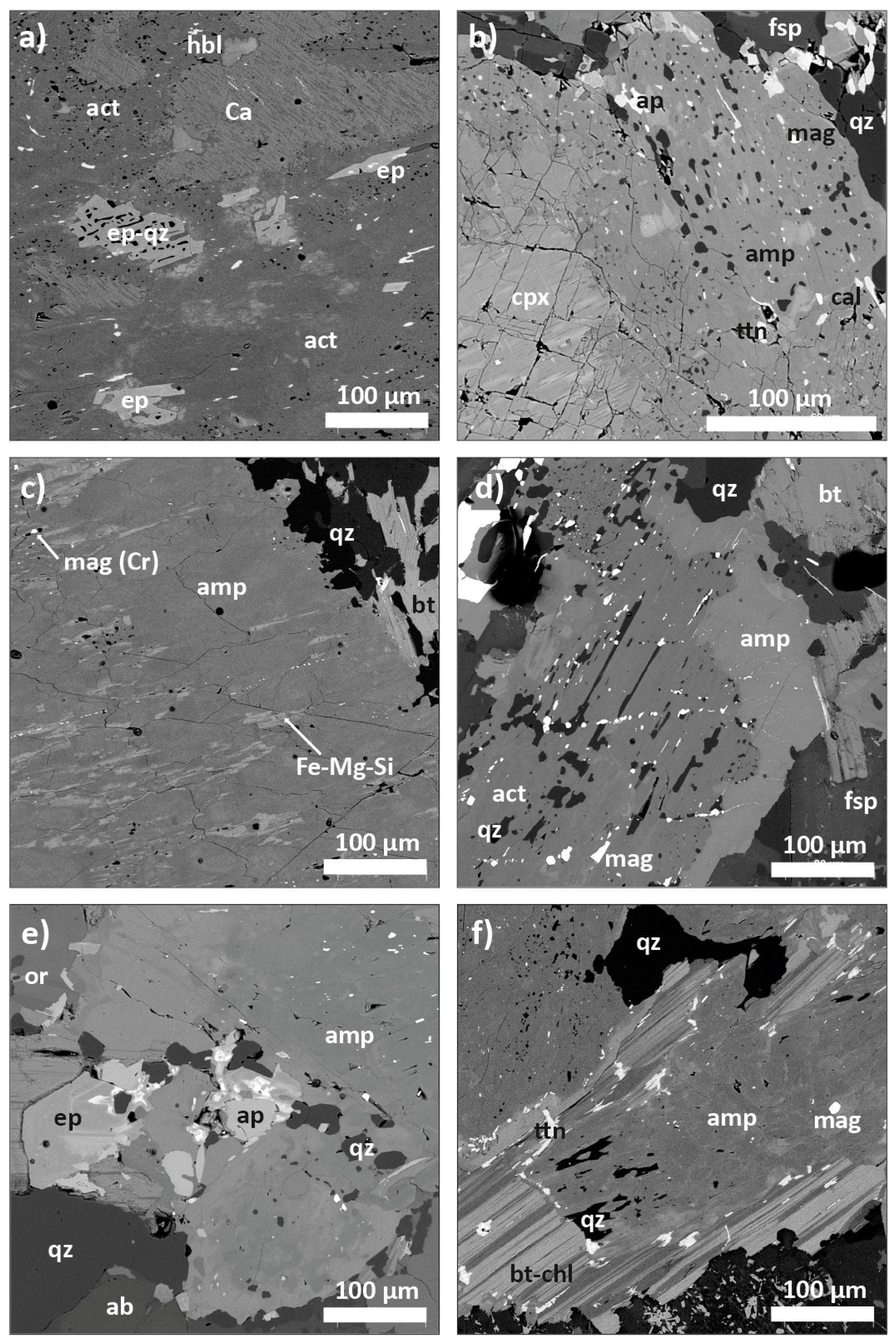

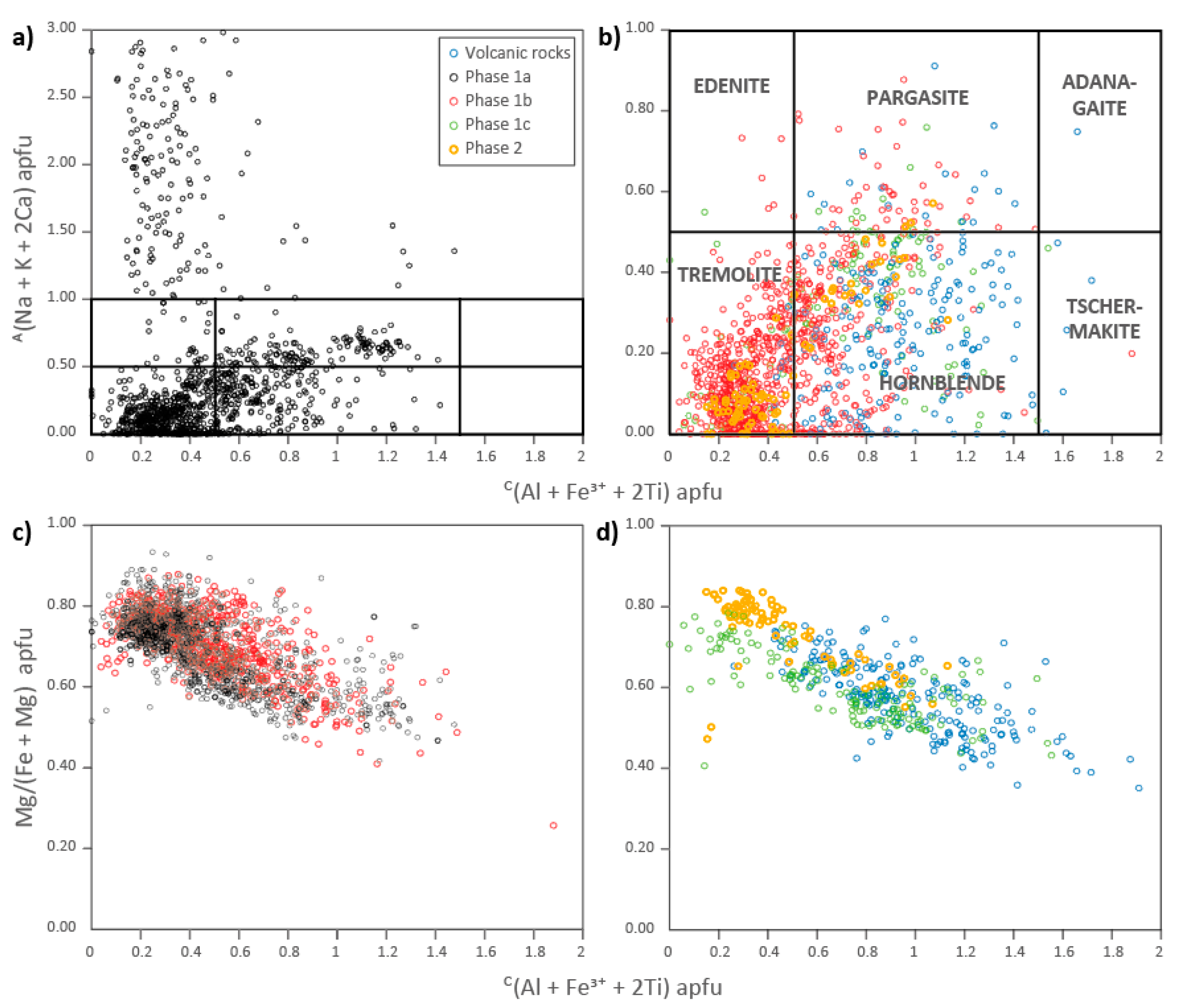

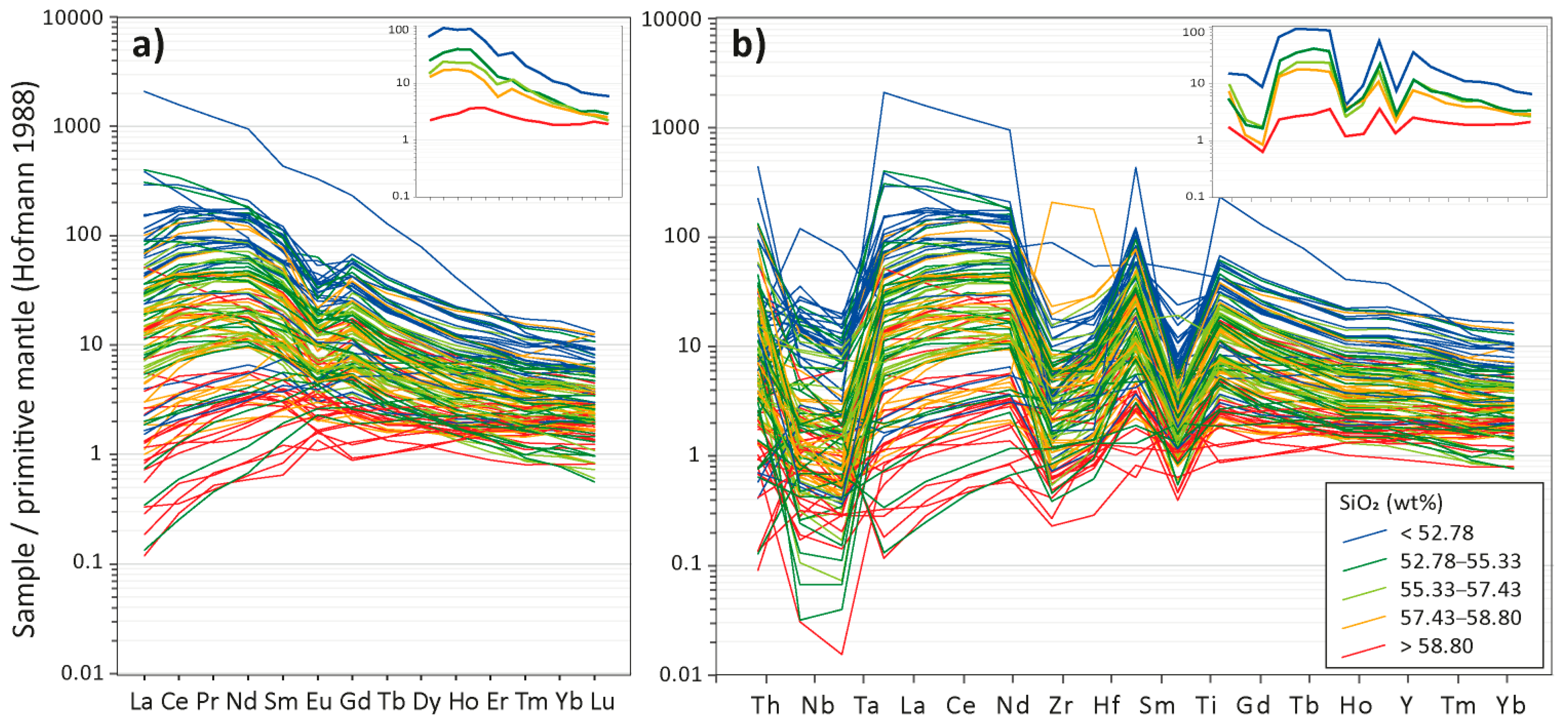
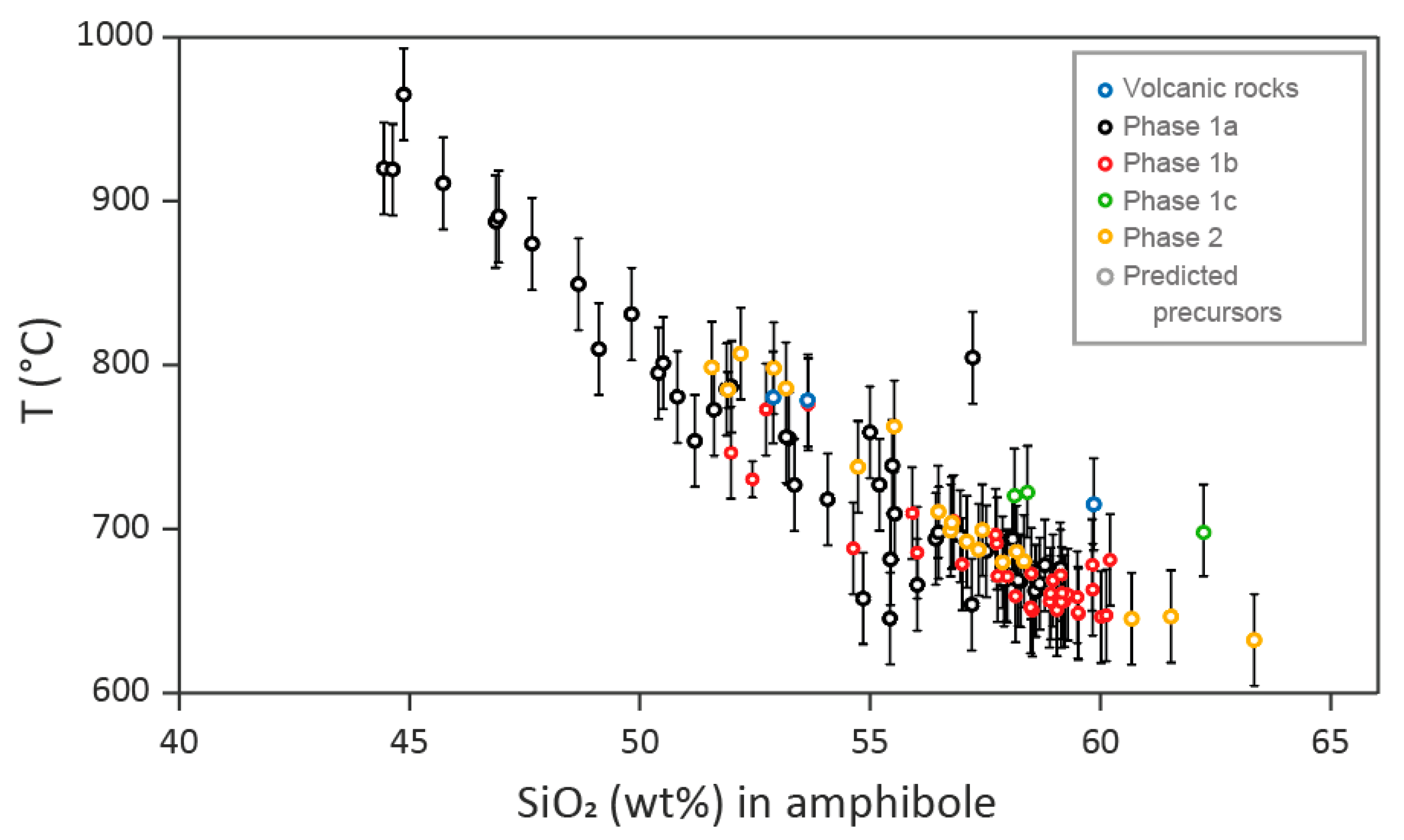
| Sample | Phase | Pl 1 | Kfs | Qz | Amp (cpx) | Bt-Chl | Ap | Ep | Mag | Ttn | Ilm | Py | Cpy | Cal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR06 | Phase 1a | 45 | 5 | 25 | 15 | 6 | 1 | 1 | 1 | Tr | Tr | 1 | ||
| TR10 | 56 | 5 | 2 | 24 | 7 | 1 | Tr 2 | 1 | 1 | Tr | 3 | |||
| TR41 | 44 | 15 | 24 | 11 | 3 | 1 | 1 | 1 | Tr | Tr | ||||
| TR43 | 65 | 5 | 10 | 11 | 4 | 1 | 1 | 1 | Tr | 1 | 1 | |||
| TR44 | 55 | 5 | 2 | 22 | 9 | 1 | 2 | 1 | Tr | Tr | 3 | |||
| TR03 | Phase 1b | 58 | 5 | 27 | 5 | 1 | 1 | Tr | Tr | 1 | Tr | 2 | ||
| TR05 | 58 | Tr | 10 | 21 | 8 | 1 | Tr | 1 | 1 | Tr | ||||
| TR07 | 75 | 5 | 12 | 3 | 1 | 2 | 1 | Tr | 1 | |||||
| TR13 | 65 | Tr | 4 | 23 | 3 | 1 | 1 | 1 | Tr | Tr | Tr | Tr | 2 | |
| TR15 | 52 | Tr | 5 | 32 | 5 | 1 | 1 | 2 | Tr | 1 | Tr | 1 | ||
| TR40 | 61 | 7 | 21 | 8 | 1 | Tr | 1 | Tr | 1 | |||||
| TR11 | Phase 1c | 55 | 13 | 2 | 20 | 1 | 1 | 3 | 5 | |||||
| TR16 | 69 | 8 | 14 | 1 | 1 | 2 | 5 | |||||||
| TR21 | 55 | 1 | 24 | 5 | 8 | 1 | 1 | 1 | Tr | Tr | Tr | 4 | ||
| TR29 3 | 48 | 28 | 7 | 13 | 1 | Tr | 2 | Tr | Tr | 1 | ||||
| TR23 | Phase 2 | 42 | 5 | 45 | 1 | 1 | Tr | Tr | Tr | 6 | ||||
| TR04 | 40 | 1 | 5 | 48 | 1 | Tr | 2 | 1 | 1 | 1 | ||||
| TR28 | Andesite | 71 | 5 | 18 | 1 | 5 | ||||||||
| TR31 | 60 | 30 | 1 | 9 | ||||||||||
| TR35 | Dacite | 45 | 27 | 13 | 4 | 1 | Tr | Tr | 10 | |||||
| TR26 | 39 | Tr | 24 | 21 | 4 | 1 | Tr | 1 | Tr | 10 | ||||
| TR32 | 59 | 20 | 15 | 5 | 1 | Tr |
| <52.78 1 | 52.78–55.33 | 55.33–57.43 | 57.43–58.80 | >58.80 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Med 2 | Std | Med | Std | Med | Std | Med | Std | Med | Std | |
| SiO2 (wt%) | 50.46 | 6.62 | 54.52 | 0.81 | 56.46 | 0.70 | 58.20 | 0.34 | 59.51 | 2.02 |
| P2O5 | 0.12 | 0.83 | 0.02 | 0.15 | 0.02 | 0.33 | 0.01 | 0.05 | 0.01 | 0.04 |
| TiO2 | 1.27 | 1.84 | 0.52 | 0.29 | 0.36 | 0.66 | 0.40 | 0.21 | 0.24 | 0.17 |
| Al2O3 | 7.87 | 2.45 | 5.59 | 2.20 | 3.74 | 0.90 | 3.16 | 2.12 | 3.18 | 2.80 |
| Cr2O3 | 0.03 | 0.10 | 0.13 | 0.21 | 0.22 | 0.19 | 0.15 | 0.19 | 0.12 | 0.33 |
| MnO | 0.34 | 0.12 | 0.27 | 0.07 | 0.27 | 0.07 | 0.28 | 0.05 | 0.28 | 0.08 |
| FeOT | 13.14 | 1.19 | 10.59 | 3.16 | 10.21 | 2.65 | 9.32 | 0.98 | 8.62 | 1.61 |
| MgO | 11.48 | 2.00 | 12.70 | 1.10 | 14.25 | 1.07 | 14.56 | 1.78 | 14.19 | 2.93 |
| CaO | 12.47 | 8.84 | 13.79 | 3.94 | 13.29 | 2.95 | 13.07 | 1.38 | 12.67 | 1.83 |
| Na2O | 0.87 | 0.27 | 0.67 | 0.22 | 0.43 | 0.11 | 0.36 | 0.52 | 0.36 | 1.04 |
| K2O | 0.71 | 0.48 | 0.33 | 0.22 | 0.15 | 0.19 | 0.19 | 0.34 | 0.06 | 0.14 |
| S (wt%) | 0.08 | 0.39 | 0.05 | 0.05 | 0.04 | 0.01 | 0.04 | 0.02 | 0.05 | 0.02 |
| Sc (ppm) | 54.29 | 25.57 | 59.77 | 25.86 | 63.02 | 23.00 | 55.28 | 26.59 | 46.55 | 34.54 |
| V | 285.01 | 64.39 | 238.11 | 84.33 | 207.05 | 80.02 | 201.95 | 85.30 | 154.26 | 104.54 |
| Cr | 183.10 | 714.99 | 885.93 | 1426.00 | 1487.05 | 1279.23 | 1026.15 | 1293.32 | 829.51 | 2260.56 |
| Co | 61.74 | 31.80 | 57.16 | 9.61 | 57.15 | 7.48 | 57.58 | 6.40 | 58.71 | 11.34 |
| Ni | 168.90 | 85.10 | 222.13 | 104.34 | 285.15 | 84.02 | 293.31 | 125.57 | 239.15 | 155.95 |
| Cu | 3.59 | 252.93 | 2.61 | 676.50 | 3.05 | 85.25 | 4.27 | 25.90 | 1.28 | 10.86 |
| Zn | 227.97 | 51.33 | 148.85 | 78.94 | 197.45 | 80.70 | 199.95 | 58.23 | 196.40 | 57.28 |
| As | 1.83 | 1.16 | 1.48 | 1.72 | 0.85 | 1.54 | 1.09 | 0.65 | 0.58 | 0.54 |
| Rb | 7.08 | 10.55 | 3.55 | 4.45 | 1.07 | 5.01 | 1.41 | 8.00 | 0.45 | 3.45 |
| Sr | 135.21 | 196.23 | 90.16 | 121.11 | 34.19 | 45.98 | 29.53 | 51.07 | 17.00 | 145.10 |
| Y | 42.13 | 31.22 | 18.48 | 19.46 | 13.72 | 12.03 | 15.01 | 15.19 | 7.49 | 6.24 |
| Zr | 43.46 | 168.41 | 30.87 | 23.23 | 24.68 | 35.12 | 33.65 | 406.73 | 9.31 | 21.22 |
| Nb | 9.10 | 14.34 | 1.07 | 3.98 | 1.33 | 2.64 | 0.74 | 1.62 | 0.60 | 0.81 |
| Mo | 0.12 | 0.28 | 0.07 | 0.10 | 0.06 | 0.22 | 0.09 | 0.18 | 0.04 | 0.28 |
| Sn | 2.61 | 2.54 | 1.11 | 3.16 | 0.81 | 2.62 | 0.78 | 1.11 | 0.72 | 0.66 |
| Ba | 77.33 | 258.51 | 28.34 | 112.94 | 9.79 | 31.85 | 13.78 | 90.06 | 6.64 | 52.89 |
| La | 41.27 | 258.85 | 15.00 | 59.73 | 8.98 | 7.93 | 7.79 | 14.61 | 1.10 | 7.21 |
| Ce | 152.39 | 499.77 | 51.11 | 136.18 | 30.96 | 32.23 | 26.28 | 52.46 | 3.77 | 18.40 |
| Pr | 22.35 | 57.68 | 8.62 | 16.88 | 5.10 | 5.29 | 4.27 | 8.46 | 0.69 | 2.78 |
| Nd | 105.37 | 218.62 | 40.05 | 67.23 | 26.16 | 25.65 | 18.54 | 38.72 | 4.15 | 13.54 |
| Sm | 22.54 | 32.44 | 7.95 | 12.23 | 5.69 | 5.44 | 4.23 | 8.00 | 1.38 | 3.15 |
| Eu | 4.43 | 9.29 | 1.72 | 1.93 | 1.08 | 0.63 | 0.84 | 0.69 | 0.45 | 0.44 |
| Gd | 17.43 | 22.90 | 5.54 | 8.22 | 4.36 | 3.92 | 4.06 | 5.40 | 1.35 | 2.43 |
| Tb | 1.92 | 2.33 | 0.67 | 0.92 | 0.52 | 0.48 | 0.55 | 0.63 | 0.20 | 0.29 |
| Dy | 9.68 | 9.65 | 4.12 | 4.43 | 2.80 | 2.53 | 3.02 | 3.25 | 1.29 | 1.51 |
| Ho | 1.61 | 1.18 | 0.70 | 0.72 | 0.54 | 0.44 | 0.57 | 0.57 | 0.27 | 0.25 |
| Er | 3.95 | 2.27 | 1.64 | 1.75 | 1.43 | 1.16 | 1.46 | 1.49 | 0.81 | 0.62 |
| Tm | 0.46 | 0.25 | 0.20 | 0.21 | 0.18 | 0.15 | 0.19 | 0.19 | 0.13 | 0.08 |
| Yb | 2.71 | 1.50 | 1.35 | 1.21 | 1.08 | 0.91 | 1.20 | 1.23 | 0.85 | 0.46 |
| Lu | 0.38 | 0.19 | 0.19 | 0.16 | 0.14 | 0.12 | 0.16 | 0.19 | 0.12 | 0.06 |
| Hf | 2.62 | 2.82 | 1.36 | 0.87 | 1.04 | 1.40 | 1.31 | 9.68 | 0.35 | 0.77 |
| Ta | 0.30 | 0.51 | 0.06 | 0.15 | 0.05 | 0.10 | 0.03 | 0.06 | 0.02 | 0.04 |
| Au | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pb | 4.41 | 4.06 | 2.66 | 2.48 | 1.27 | 0.60 | 1.70 | 12.57 | 0.78 | 1.45 |
| Bi | 0.11 | 0.16 | 0.04 | 0.12 | 0.04 | 0.21 | 0.04 | 0.03 | 0.03 | 0.02 |
| Th | 1.22 | 7.95 | 0.41 | 2.62 | 0.74 | 1.00 | 0.54 | 2.33 | 0.13 | 1.04 |
| U | 0.77 | 2.56 | 0.24 | 1.16 | 0.24 | 0.19 | 0.19 | 1.39 | 0.07 | 0.79 |
| Field Designation | Mineralogy | Mineralogy and Chemistry | Final |
|---|---|---|---|
| Diorite | Phase 1a | Granodiorite | Granodiorite intrusive phase (1a–1c) |
| Phase 1c | |||
| Phase 1b | Diorite | Diorite intrusive phase (1b) | |
| Gabbro | Phase 2 (TR04) | Gabbro | Gabbro intrusive phase |
| Phase 2 (TR23) | Basalt | Volcanic hostrock | |
| Dacite | (thin-grained and altered) | Dacite | |
| Andesite | Basalt-Andesite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu, L.; MacDonald, F. Petrography and Geochemistry of the Intrusive Rocks at the Diorite-Hosted Regnault Au Mineralization. Minerals 2022, 12, 128. https://doi.org/10.3390/min12020128
Mathieu L, MacDonald F. Petrography and Geochemistry of the Intrusive Rocks at the Diorite-Hosted Regnault Au Mineralization. Minerals. 2022; 12(2):128. https://doi.org/10.3390/min12020128
Chicago/Turabian StyleMathieu, Lucie, and Francis MacDonald. 2022. "Petrography and Geochemistry of the Intrusive Rocks at the Diorite-Hosted Regnault Au Mineralization" Minerals 12, no. 2: 128. https://doi.org/10.3390/min12020128






