Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines
Abstract
:1. Introduction
1.1. Coagulation System Activation in Different Diseases
1.2. Hypercoagulability and Viral Infections
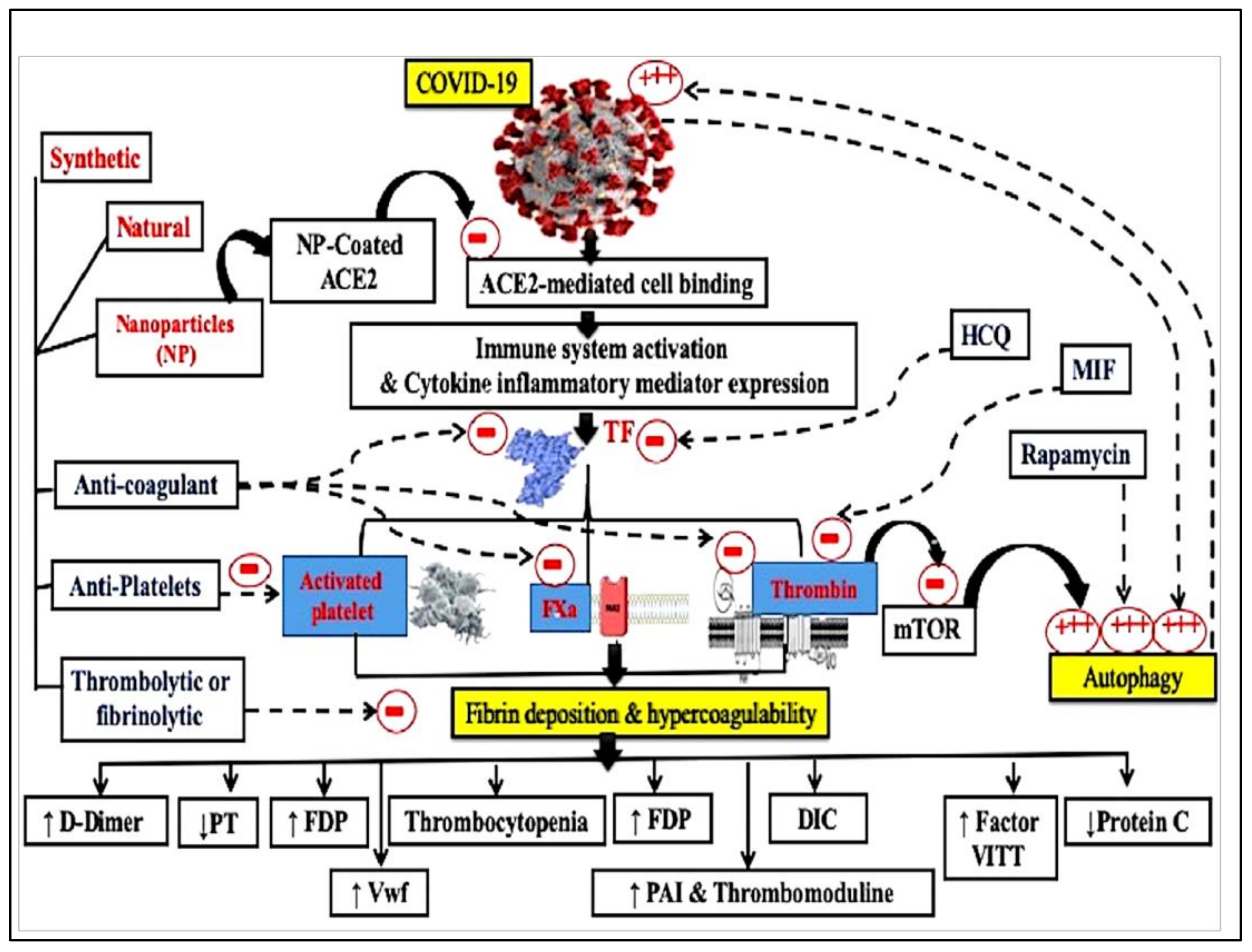
1.3. Hypercoagulability Markers in COVID-19
1.4. Fibrinolysis in COVID-19 Patients
1.5. Therapeutic Approaches for Hypercoagulability in COVID-19
1.6. Natural Products as Anticoagulants
| Mechanism of Action | Natural Source | Active Constituents | References | |
|---|---|---|---|---|
| Anticoagulant drugs | 1-TF inhibitors | Chaenomeles sinensis | Hovertrichoside, luteolin-7-O-β-D-glucuronide, hyperin, avicularin and quercetin | [91] |
| Ligustici chuanxiong | Ligustrazine | [87] | ||
| Eriobotrya japonica Lindley | Sesquiterpene glycoside | [92] | ||
| Beans and grain | α-Zearalanol | [87] | ||
| 2-Inhibitors of the intrinsic and extrinsic coagulation pathways | The green algae Monostroma arcticum | Polysaccharide | [87] | |
| Polygala fallax Hesml. | Saponins | [87] | ||
| Rhododendron brachycarpum | Hyperoside, | [93] | ||
| Umbilicaria esculenta | Polysaccharide | [94] | ||
| Withania somnifera | Withaferin A | [95] | ||
| Scutellaria baicalensis Georgi | Wogonin and wogonoside | [96] | ||
| Erigeron canadensis L. | Polyphenolic-polysaccharide preparation | [97] | ||
| Codium vermilara | Polysaccharide | [98] | ||
| Crassocephalum crepidioides | Crude extract | [99] | ||
| Anti-platelet aggregation drugs | 1-Acting by variable mechanisms | Andrographis paniculata | Andrographolide | [100] |
| Bupleurumfalcatum | Bupleurumin | [101] | ||
| Salvia milthorriza Bunge | Tanshinone IIA | [102] | ||
| Abies webbiana, parsley, Nigella sativa | Crude extract | [103,104,105] | ||
| 2-Inhibitors of platelet membrane receptors | Spatholobus suberectus, garlic | Crude extract | [106,107] | |
| Rabdosia japonica var. glaucocalyx | Glaucocalyxin A | [108] | ||
| Salvia miltiorrhiza | Salvianolic acid B | [109] | ||
| Garcinia nervosa var. pubescens King | Flavonoids | [110] | ||
| Erylus formosus | Eryloside F | [111] | ||
| Piper longum | Piperlongumine | [112] | ||
| Licania pittieri | Pomolic acid | [113] | ||
| Polygonum multiflorum | Tetrahydroxystilbene glucoside | [114] | ||
| Agkistrodon acutus Venom | Tripeptide | [115] | ||
| Cruciferous vegetables | Indole-3-carbinol | [116] | ||
| Goniothalamus species | Essential oils | [117] | ||
| Ligusticam wallichii Franch | Tetramethyl pyrazine | [118] | ||
| Agrimonia pilosa, Toona sinensis | Crude extract | [87] | ||
| Rhus verniciflua Stokes | Isomaltol and pentagalloyl glucose | [119] | ||
| 3-Impacting on nucleotide system. | Cordyceps militaris | Cordycepin | [120] | |
| Ginkgo biloba | Ginkgolide C, quercetin | [121] | ||
| Oligoporus tephroleucus | Oligoporin A | [122] | ||
| 4-Inhibitors of platelet granules secretion. | Saffron | Crocetin | [123] | |
| Black soybean | Crude extract | [124] | ||
| Magnolia bark | Magnolol | [125] | ||
| Solanum lycopersicum | Guanosine | [126] | ||
| Ligustici Chuanxiong | Ligustrazine ferulate, | [87] | ||
| Rhizoma Curcumae | Curdione | [127] | ||
| 5-Impacting on arachidonic acid system | Green tea leaves | Epigallocatechin-3-gallate | [128] | |
| Zizyphus jujube | Jujuboside B | [129] | ||
| Sorghum vinegar | Alditol and monosaccharide | [130] | ||
| Magnolia obovate | Diacetylated obovatol | [131] | ||
| Artemisia princeps Pampanini | Crude extract, eupatilin, and jaceosidin | [132] | ||
| Grape fruits and oranges | Hesperetin | [133] | ||
| Betel leaf | Hydroxychavicol | [134] | ||
| Stephaniae tetrandrae | Tetrandrine and fangchinoline | [135] | ||
| Uncaria sinensis (Oliv.) Havil. | Isorhynchophylline | [87] | ||
| Caesalpinia sappan L. | Ethyl acetate extract | [87] | ||
| Cornus officinalis Sieb. et Zucc | Morroniside | [87] | ||
| Pleurothyrium cinereum, Ocotea macrophylla and Nectandra amazonum | Neolignans | [136] | ||
| Zingiber mioga Roscoe | Crude extracts | [137] | ||
| White ginseng | Ginsenoside Rk1 | [138] | ||
1.7. Nanotechnology for Future Treatment of COVID-19
1.8. Nanoparticle-Loaded Anticoagulants
1.9. COVID-19 Vaccine Loaded Nanoparticles
1.10. Coagulation System Activation and COVID-19 Vaccines
2. Conclusions
Compliance with Ethical Standards
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Khan, R.A.; Alhowail, A.H.; Alqasoumi, A.; Sajid, S.M.; Mohammed, A.M.; Alsharidah, M.; Al Rugaie, O.; Mousa, A.M. Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model. Nanotechnol. Rev. 2021, 11, 266–283. [Google Scholar] [CrossRef]
- Ferrari, M.F.; Raizada, M.K.; Fior-Chadi, D.R. Nicotine modulates the renin-angiotensin system of cultured neurons and glial cells from cardiovascular brain areas of Wistar Kyoto and spontaneously hypertensive rats. J. Mol. Neurosci. 2007, 33, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Schnittler, H.J.; Feldmann, H. Viral hemorrhagic fever—A vascular disease? Thromb. Haemost. 2003, 89, 967–972. [Google Scholar]
- Zhou, X.; Cheng, Z.; Luo, L.; Zhu, Y.; Lin, W.; Ming, Z.; Chen, W.; Hu, Y. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb. Res. 2021, 201, 23–29. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Key, N.S.; Vercellotti, G.M.; Winkelmann, J.C.; Moldow, C.F.; Goodman, J.L.; Esmon, N.L.; Esmon, C.T.; Jacob, H.S. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc. Natl. Acad. Sci. USA 1990, 87, 7095–7099. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Scharrer, I. Procoagulant activity during viral infections. Front. Biosci. 2018, 23, 1060–1081. [Google Scholar] [CrossRef] [Green Version]
- De Caterina, R.; D’Ugo, E.; Libby, P. Inflammation and thrombosis-testing the hypothesis with anti-inflammatory drug trials. Thromb. Haemost. 2016, 116, 1012–1021. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell. Mol. Immunol. 2016, 13, 432–442. [Google Scholar] [CrossRef]
- Neumann, F.J.; Marx, N.; Gawaz, M.; Brand, K.; Ott, I.; Rokitta, C.; Sticherling, C.; Meinl, C.; May, A.; Schomig, A. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 1997, 95, 2387–2394. [Google Scholar] [CrossRef]
- Van Gorp, E.C.; Suharti, C.; ten Cate, H.; Dolmans, W.M.; van der Meer, J.W.; ten Cate, J.W.; Brandjes, D.P. Review: Infectious diseases and coagulation disorders. J. Infect. Dis. 1999, 180, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, A.A.H.; Mohammed, H.A.; Khan, R.A.; Singh, V.; Bouazzaoui, A.; Yusuf, M.; Akhtar, N.; Khan, M.; Al-Subaiyel, A.; Mohammed, S.A.A.; et al. Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity. Nanotechnol. Rev. 2021, 10, 1493–1559. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alsowinea, A.F. Approved and marketed nanoparticles for disease targeting and applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the blood coagulation system. Part I: Benefits of nanotechnology. Nanomedicine 2013, 8, 773–784. [Google Scholar] [CrossRef]
- Lorenzano, S.; Inglese, M.; Koudriavtseva, T. Editorial: Role of Coagulation Pathways in Neurological Diseases. Front. Neurol. 2019, 10, 791. [Google Scholar] [CrossRef]
- Davalos, D.; Mahajan, K.R.; Trapp, B.D. Brain fibrinogen deposition plays a key role in MS pathophysiology—Yes. Mult. Scler. 2019, 25, 1434–1435. [Google Scholar] [CrossRef] [Green Version]
- Plantone, D.; Inglese, M.; Salvetti, M.; Koudriavtseva, T. Corrigendum: A Perspective of Coagulation Dysfunction in Multiple Sclerosis and in Experimental Allergic Encephalomyelitis. Front. Neurol. 2019, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Levi, M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb. Res. 2018, 164 (Suppl. S1), S77–S81. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Hammad, M.A.; Walker, L.A.; Ashfaq, M.K. Tissue factor dependent liver injury causes release of retinoid receptors (RXR-alpha and RAR-alpha) as lipid droplets. Biochem. Biophys. Res. Commun. 2011, 410, 146–151. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Helal, G.K.; El-Sayed, E.M.; Alhowail, A.H.; Mansour, A.M.; Alharbi, K.S.; Amin, E.; Allam, S.; Salama, S.A.; Saad, A.S. Silencing of tissue factor by antisense deoxyoligonucleotide mitigates thioacetamide-induced liver injury. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 1887–1898. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Helal, G.K.; El-Sayed, E.M.; Saad, A.S. Carbon tetrachloride-induced liver injury in mice is tissue factor dependent. Environ. Toxicol. Pharmacol. 2015, 39, 1199–1205. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Hammad, M.A.; Walker, L.A.; Ashfaq, M.K. Silencing of tissue factor by antisense deoxyoligonucleotide prevents monocrotaline/LPS renal injury in mice. Arch. Toxicol. 2011, 85, 1245–1256. [Google Scholar] [CrossRef]
- Mahmoud, N.I.; Messiha, B.A.S.; Salehc, I.G.; Abo-Saif, A.A.; Abdel-Bakky, M.S. Interruption of platelets and thrombin function as a new approach against liver fibrosis induced experimentally in rats. Life Sci. 2019, 231, 116522. [Google Scholar] [CrossRef]
- Ewees, M.G.; Messiha, B.A.S.; Abo-Saif, A.A.; Bayoumi, A.M.A.; Abdel-Bakky, M.S. Interference with Coagulation Cascade as a Novel Approach to Counteract Cisplatin-Induced Acute Tubular Necrosis; an Experimental Study in Rats. Front. Pharmacol. 2018, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- DeFeo, K.; Hayes, C.; Chernick, M.; Ryn, J.V.; Gilmour, S.K. Use of dabigatran etexilate to reduce breast cancer progression. Cancer Biol. Ther. 2010, 10, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasimuzzaman, M.; Malik, P. Role of the coagulation system in the pathogenesis of sickle cell disease. Blood Adv. 2019, 3, 3170–3180. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef]
- Tal, S.; Spectre, G.; Kornowski, R.; Perl, L. Venous Thromboembolism Complicated with COVID-19: What Do We Know So Far? Acta Haematol. 2020, 143, 417–424. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Paessler, S.; Walker, D.H. Pathogenesis of the viral hemorrhagic fevers. Annu. Rev. Pathol. 2013, 8, 411–440. [Google Scholar] [CrossRef]
- Falasca, L.; Agrati, C.; Petrosillo, N.; Di Caro, A.; Capobianchi, M.R.; Ippolito, G.; Piacentini, M. Molecular mechanisms of Ebola virus pathogenesis: Focus on cell death. Cell Death Differ. 2015, 22, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Su, F.; Lin, X.J.; Dai, B.; Kong, L.F.; Zhao, H.W.; Kang, J. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1). Thromb. Res. 2011, 127, 198–201. [Google Scholar] [CrossRef]
- Borges, A.H.; O’Connor, J.L.; Phillips, A.N.; Baker, J.V.; Vjecha, M.J.; Losso, M.H.; Klinker, H.; Lopardo, G.; Williams, I.; Lundgren, J.D.; et al. Factors associated with D-dimer levels in HIV-infected individuals. PLoS ONE 2014, 9, e90978. [Google Scholar] [CrossRef]
- Assinger, A. Platelets and infection—An emerging role of platelets in viral infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human blood platelets and viruses: Defense mechanism and role in the removal of viral pathogens. Thromb. J. 2018, 16, 16. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Wong, R.S.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.; Wong, C.K.; Chan, P.K.; Ng, M.H.; Yu, L.M.; Hui, D.S.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Ng, M.H.; Li, C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2005, 10, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Qu, R.; Ling, Y.; Zhang, Y.H.; Wei, L.Y.; Chen, X.; Li, X.M.; Liu, X.Y.; Liu, H.M.; Guo, Z.; Ren, H.; et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020, 92, 1533–1541. [Google Scholar] [CrossRef]
- Polycarpou, A.; Howard, M.; Farrar, C.A.; Greenlaw, R.; Fanelli, G.; Wallis, R.; Klavinskis, L.S.; Sacks, S. Rationale for targeting complement in COVID-19. EMBO Mol. Med. 2020, 12, e12642. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Q.; Florence, J.M.; Jung, B.G.; Kurdowska, A.K.; Samten, B.; Idell, S.; Tang, H. Influenza Virus Infection Induces ZBP1 Expression and Necroptosis in Mouse Lungs. Front. Cell. Infect. Microbiol. 2019, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, S.; Biswas, I.; Ahmed, R.; Khan, G.A. Hypoxia induced up-regulation of tissue factor is mediated through extracellular RNA activated Toll-like receptor 3-activated protein 1 signalling. Blood Cells Mol. Dis. 2020, 84, 102459. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Huang, M.; Li, D.; Tang, N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis 2020, 49, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Escher, R.; Breakey, N.; Lammle, B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020, 190, 62. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- Agrawal, A.; Zhuo, H.; Brady, S.; Levitt, J.; Steingrub, J.; Siegel, M.D.; Soto, G.; Peterson, M.W.; Chesnutt, M.S.; Matthay, M.A.; et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: Results from two clinical trials. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L634–L639. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Ware, L.B.; White, K.E.; Cross, M.T.; Matthay, M.A.; Olman, M.A. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L20–L28. [Google Scholar] [CrossRef] [Green Version]
- Sapru, A.; Calfee, C.S.; Liu, K.D.; Kangelaris, K.; Hansen, H.; Pawlikowska, L.; Ware, L.B.; Alkhouli, M.F.; Abbott, J.; Matthay, M.A.; et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med. 2015, 41, 470–478. [Google Scholar] [CrossRef]
- Thompson, B.T. Acute respiratory distress syndrome in another 50 years: Historical footnote or persistent malady? Curr. Opin. Crit. Care 2017, 23, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A.; Parsons, P.E.; Thompson, B.T.; Januzzi, J.L.; Eisner, M.D.; The National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit. Care Med. 2007, 35, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Bastarache, J.A.; Wang, L.; Geiser, T.; Wang, Z.; Albertine, K.H.; Matthay, M.A.; Ware, L.B. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 2007, 62, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Becker, R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef]
- Authorization, F.a.D.A.i.a.e.u. Janssen COVID-19 Vaccine Frequently Asked Questions. 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine-frequently-asked-questions (accessed on 27 November 2021).
- Agency, E.M. Signal Assessment Report on Embolic and Thrombotic Events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant])–COVID-19 Vaccine AstraZeneca (Other Viral Vaccines). 2021. Available online: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant_en.pdf (accessed on 27 November 2021).
- Talotta, R.; Robertson, E.S. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: The straw that breaks the camel’s back? Cytokine Growth Factor Rev. 2021, 60, 52–60. [Google Scholar] [CrossRef]
- Levy, J.H.; Iba, T.; Olson, L.B.; Corey, K.M.; Ghadimi, K.; Connors, J.M. COVID-19: Thrombosis, thromboinflammation, and anticoagulation considerations. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 29–35. [Google Scholar] [CrossRef]
- Kwaan, H.C. From fibrinolysis to the plasminogen-plasmin system and beyond: A remarkable growth of knowledge, with personal observations on the history of fibrinolysis. Semin. Thromb. Hemost. 2014, 40, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Kwaan, H.C.; Lindholm, P.F. The Central Role of Fibrinolytic Response in COVID-19-A Hematologist’s Perspective. Int. J. Mol. Sci. 2021, 22, 1283. [Google Scholar] [CrossRef]
- Komissarov, A.A.; Rahman, N.; Lee, Y.C.G.; Florova, G.; Shetty, S.; Idell, R.; Ikebe, M.; Das, K.; Tucker, T.A.; Idell, S. Fibrin turnover and pleural organization: Bench to bedside. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L757–L768. [Google Scholar] [CrossRef]
- Tucker, T.; Idell, S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin. Thromb. Hemost. 2013, 39, 373–381. [Google Scholar] [CrossRef]
- Schmitt, F.C.F.; Manolov, V.; Morgenstern, J.; Fleming, T.; Heitmeier, S.; Uhle, F.; Al-Saeedi, M.; Hackert, T.; Bruckner, T.; Schochl, H.; et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: Results of an observational pilot study. Ann. Intensive Care 2019, 9, 19. [Google Scholar] [CrossRef]
- Panigada, M.; Zacchetti, L.; L’Acqua, C.; Cressoni, M.; Anzoletti, M.B.; Bader, R.; Protti, A.; Consonni, D.; D’Angelo, A.; Gattinoni, L. Assessment of Fibrinolysis in Sepsis Patients with Urokinase Modified Thromboelastography. PLoS ONE 2015, 10, e0136463. [Google Scholar] [CrossRef] [Green Version]
- Bhandary, Y.P.; Shetty, S.K.; Marudamuthu, A.S.; Ji, H.L.; Neuenschwander, P.F.; Boggaram, V.; Morris, G.F.; Fu, J.; Idell, S.; Shetty, S. Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am. J. Pathol. 2013, 183, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Puthusseri, B.; Marudamuthu, A.; Tiwari, N.; Fu, J.; Idell, S.; Shetty, S. Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L783–L796. [Google Scholar] [CrossRef] [Green Version]
- Gouda, M.M.; Shaikh, S.B.; Bhandary, Y.P. Inflammatory and Fibrinolytic System in Acute Respiratory Distress Syndrome. Lung 2018, 196, 609–616. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Jaimes, F.; De La Rosa, G.; Morales, C.; Fortich, F.; Arango, C.; Aguirre, D.; Munoz, A. Unfractioned heparin for treatment of sepsis: A randomized clinical trial (The HETRASE Study). Crit. Care Med. 2009, 37, 1185–1196. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Galluccio, F.; Ergonenc, T.; Garcia Martos, A.; Allam, A.E.; Perez-Herrero, M.; Aguilar, R.; Emmi, G.; Spinicci, M.; Terrancle Juan, I.; Fajardo-Perez, M. Treatment algorithm for COVID-19: A multidisciplinary point of view. Clin. Rheumatol. 2020, 39, 2077–2084. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.; Meijers, J.C.; Brandjes, D.P.; van Gorp, E.C. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. In vivo thrombus formation. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 12–17. [Google Scholar] [CrossRef]
- Chen, C.; Yang, F.Q.; Zhang, Q.; Wang, F.Q.; Hu, Y.J.; Xia, Z.N. Natural Products for Antithrombosis. Evid.-Based Complement. Altern. Med. 2015, 2015, 876426. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, E.; Guzmán, L.; Alarcón, M.; Moore, R.; Palomo, I. Thrombolytic/fibrinolytic mechanism of natural products. In Fibrinolysis and Thrombolysis; IntechOpen Ltd.: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, T.A.; Andryukov, B.G.; Makarenkova, I.D.; Zaporozhets, T.S.; Besednova, N.N.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Shchelkanov, M.Y. The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19. Molecules 2021, 26, 2618. [Google Scholar] [CrossRef]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Pharm. Res. 2002, 25, 842–850. [Google Scholar] [CrossRef]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory sesquiterpene glycoside from Eriobotrya japonica. Arch. Pharm. Res. 2004, 27, 619–623. [Google Scholar] [CrossRef]
- Ku, S.-K.; Yoo, H.; Zhou, W.; Na, M.; Bae, J.-S. Antiplatelet activities of hyperosidein vitroandin vivo. Anim. Cells Syst. 2014, 18, 204–209. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, J.; Yao, S.; Zhang, S.; Yan, J.; Wang, H.; Chen, Y. Study on the antithrombotic activity of Umbilicaria esculenta polysaccharide. Carbohydr. Polym. 2014, 105, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A. Vasc. Pharm. 2014, 60, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Antithrombotic activities of wogonin and wogonoside via inhibiting platelet aggregation. Fitoterapia 2014, 98, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk, I.; Czerchawski, L.; Kuliczkowski, W.; Karolko, B.; Pilecki, W.; Witkiewicz, W.; Gancarz, R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb. Res. 2011, 127, 328–340. [Google Scholar] [CrossRef]
- Fernandez, P.V.; Quintana, I.; Cerezo, A.S.; Caramelo, J.J.; Pol-Fachin, L.; Verli, H.; Estevez, J.M.; Ciancia, M. Anticoagulant activity of a unique sulfated pyranosic (1->3)-beta-L-arabinan through direct interaction with thrombin. J. Biol. Chem. 2013, 288, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayodele, O.O.; Onajobi, F.D.; Osoniyi, O. In vitro anticoagulant effect of Crassocephalum crepidioides leaf methanol extract and fractions on human blood. J. Exp. Pharmacol. 2019, 11, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Amroyan, E.; Gabrielian, E.; Panossian, A.; Wikman, G.; Wagner, H. Inhibitory effect of andrographolide from Andrographis paniculata on PAF-induced platelet aggregation. Phytomedicine 1999, 6, 27–31. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yun-Choi, H.S. Platelet anti-aggregating activities of bupleurumin from the aerial parts of Bupleurum falcatum. Arch. Pharm. Res. 2007, 30, 561–564. [Google Scholar] [CrossRef]
- Maione, F.; De Feo, V.; Caiazzo, E.; De Martino, L.; Cicala, C.; Mascolo, N. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J. Ethnopharmacol. 2014, 155, 1236–1242. [Google Scholar] [CrossRef]
- Yasin, M.; Hussain Janbaz, K.; Imran, I.; Gilani, A.U.; Bashir, S. Pharmacological studies on the antispasmodic, bronchodilator and anti-platelet activities of Abies webbiana. Phytother. Res. 2014, 28, 1182–1187. [Google Scholar] [CrossRef]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Legrand, C.; Lafeve, F.F.; Mekhfi, H. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J. Ethnopharmacol. 2009, 125, 170–174. [Google Scholar] [CrossRef]
- Ansari, V.; Mujahid, M.; Siddiqui, H.; Dixit, R.; Singh, K. In vitro study for antiplatelet activity of ‘Kalonji’(Nigella sativa) extracts using aspirin as standard. J. Chem. Pharm. Res. 2016, 8, 182–185. [Google Scholar]
- Lee, B.J.; Jo, I.Y.; Bu, Y.; Park, J.W.; Maeng, S.; Kang, H.; Jang, W.; Hwang, D.S.; Lee, W.; Min, K.; et al. Antiplatelet effects of Spatholobus suberectus via inhibition of the glycoprotein IIb/IIIa receptor. J. Ethnopharmacol. 2011, 134, 460–467. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012, 91, 1275–1280. [Google Scholar] [CrossRef]
- Zhang, B.; Long, K. Effects of glaucocalyxin A on aggregation and cAMP levels of rabbit platelets in vitro. Zhongguo Yao Li Xue Bao 1993, 14, 347–350. [Google Scholar]
- Ma, C.; Yao, Y.; Yue, Q.X.; Zhou, X.W.; Yang, P.Y.; Wu, W.Y.; Guan, S.H.; Jiang, B.H.; Yang, M.; Liu, X.; et al. Differential proteomic analysis of platelets suggested possible signal cascades network in platelets treated with salvianolic acid B. PLoS ONE 2011, 6, e14692. [Google Scholar] [CrossRef]
- Jalil, J.; Jantan, I.; Ghani, A.A.; Murad, S. Platelet-activating factor (PAF) antagonistic activity of a new biflavonoid from Garcinia nervosa var. pubescens King. Molecules 2012, 17, 10893–10901. [Google Scholar] [CrossRef]
- Stead, P.; Hiscox, S.; Robinson, P.S.; Pike, N.B.; Sidebottom, P.J.; Roberts, A.D.; Taylor, N.L.; Wright, A.E.; Pomponi, S.A.; Langley, D. Eryloside F, a novel penasterol disaccharide possessing potent thrombin receptor antagonist activity. Bioorganic Med. Chem. Lett. 2000, 10, 661–664. [Google Scholar] [CrossRef]
- Iwashita, M.; Oka, N.; Ohkubo, S.; Saito, M.; Nakahata, N. Piperlongumine, a constituent of Piper longum L., inhibits rabbit platelet aggregation as a thromboxane A2 receptor antagonist. Eur. J. Pharmacol. 2007, 570, 38–42. [Google Scholar] [CrossRef]
- Alvarado-Castillo, C.; Estrada, O.; Carvajal, E. Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets. Phytomedicine 2012, 19, 484–487. [Google Scholar] [CrossRef]
- Xiang, K.; Liu, G.; Zhou, Y.J.; Hao, H.Z.; Yin, Z.; He, A.D.; Da, X.W.; Xiang, J.Z.; Wang, J.L.; Ming, Z.Y. 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) attenuates human platelet aggregation, secretion and spreading in vitro. Thromb. Res. 2014, 133, 211–217. [Google Scholar] [CrossRef]
- Kong, Y.; Huo, J.L.; Xu, W.; Xiong, J.; Li, Y.M.; Wu, W.T. A novel anti-platelet aggregation tripeptide from Agkistrodon acutus venom: Isolation and characterization. Toxicon 2009, 54, 103–109. [Google Scholar] [CrossRef]
- Park, M.K.; Rhee, Y.H.; Lee, H.J.; Lee, E.O.; Kim, K.H.; Park, M.J.; Jeon, B.H.; Shim, B.S.; Jung, C.H.; Choi, S.H.; et al. Antiplatelet and antithrombotic activity of indole-3-carbinol in vitro and in vivo. Phytother. Res. 2008, 22, 58–64. [Google Scholar] [CrossRef]
- Moharam, B.A.; Jantan, I.; Ahmad, F.; Jalil, J. Antiplatelet aggregation and platelet activating factor (PAF) receptor antagonistic activities of the essential oils of five Goniothalamus species. Molecules 2010, 15, 5124–5138. [Google Scholar] [CrossRef] [Green Version]
- Sheu, J.-R.; Kan, Y.-C.; Hung, W.-C.; Ko, W.-C.; Yen, M.-H. Mechanisms Involved in the Antiplatelet Activity of Tetramethylpyrazine in Human Platelets. Thromb. Res. 1997, 88, 259–270. [Google Scholar] [CrossRef]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef]
- Lee, D.H.; Kwon, H.W.; Kim, H.H.; Lim, D.H.; Nam, G.S.; Shin, J.H.; Kim, Y.Y.; Kim, J.L.; Lee, J.J.; Kwon, H.K.; et al. Cordycepin-enriched WIB801C from Cordyceps militaris inhibits ADP-induced [Ca2+]i mobilization and fibrinogen binding via phosphorylation of IP 3R and VASP. Arch. Pharm. Res. 2015, 38, 81–97. [Google Scholar] [CrossRef]
- Cho, H.J.; Shon, Y.H.; Nam, K.S. Ginkgolide C inhibits platelet aggregation in cAMP- and cGMP-dependent manner by activating MMP-9. Biol. Pharm. Bull. 2007, 30, 2340–2344. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Oh, W.J.; Kim, M.J.; Kim, T.H.; Cho, J.Y.; Park, H.J.; Lee, I.K.; Kim, S.; Kim, G.S.; Kim, S.K.; et al. Mechanism of anti-platelet activity of Oligoporus tephroleucus oligoporin A: Involvement of extracellular signal-regulated kinase phosphorylation and cyclic nucleotide elevation. Platelets 2012, 23, 376–385. [Google Scholar] [CrossRef]
- Yang, L.; Qian, Z.; Yang, Y.; Sheng, L.; Ji, H.; Zhou, C.; Kazi, H.A. Involvement of Ca2+ in the inhibition by crocetin of platelet activity and thrombosis formation. J. Agric. Food Chem. 2008, 56, 9429–9433. [Google Scholar] [CrossRef]
- Kim, K.; Lim, K.M.; Kim, C.W.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Noh, J.Y.; Bae, O.N.; Shin, S.; Chung, J.H. Black soybean extract can attenuate thrombosis through inhibition of collagen-induced platelet activation. J. Nutr. Biochem. 2011, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Tsai, W.-J.; Chou, C.-J.; Chen, C.-F. Magnolol inhibits collagen-induced platelet serotonin release. Thromb. Res. 1995, 78, 265–270. [Google Scholar] [CrossRef]
- Fuentes, E.; Alarcon, M.; Astudillo, L.; Valenzuela, C.; Gutierrez, M.; Palomo, I. Protective mechanisms of guanosine from Solanum lycopersicum on agonist-induced platelet activation: Role of sCD40L. Molecules 2013, 18, 8120–8135. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, X.; Xu, D.J.; Chen, X.H.; Chen, F.H. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb. Res. 2012, 130, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, Y.J.; Kim, H.H.; Cho, H.J.; Ryu, J.H.; Rhee, M.H.; Park, H.J. Inhibitory effects of epigallocatechin-3-gallate on microsomal cyclooxygenase-1 activity in platelets. Biomol. Ther. 2013, 21, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.J.; Lee, S.Y.; Kang, S.S.; Jung, Y.S. Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytother. Res. 2013, 27, 829–834. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Fan, J. Alditols and monosaccharides from sorghum vinegar can attenuate platelet aggregation by inhibiting cyclooxygenase-1 and thromboxane-A2 synthase. J. Ethnopharmacol. 2014, 155, 285–292. [Google Scholar] [CrossRef]
- Yu, J.Y.; Lee, J.J.; Jung, J.K.; Min, Y.K.; Ma, J.Y.; Kim, T.J.; Lee, M.Y.; Yun, Y.P. Anti-platelet activity of diacetylated obovatol through regulating cyclooxygenase and lipoxygenase activities. Arch. Pharm. Res. 2012, 35, 2191–2198. [Google Scholar] [CrossRef]
- Ryu, R.; Jung, U.J.; Kim, H.J.; Lee, W.; Bae, J.S.; Park, Y.B.; Choi, M.S. Anticoagulant and Antiplatelet Activities of Artemisia princeps Pampanini and Its Bioactive Components. Prev. Nutr. Food Sci. 2013, 18, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.R.; Han, X.H.; Zhang, Y.H.; Lee, J.J.; Lim, Y.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-gamma2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef]
- Chang, M.C.; Uang, B.J.; Tsai, C.Y.; Wu, H.L.; Lin, B.R.; Lee, C.S.; Chen, Y.J.; Chang, C.H.; Tsai, Y.L.; Kao, C.J.; et al. Hydroxychavicol, a novel betel leaf component, inhibits platelet aggregation by suppression of cyclooxygenase, thromboxane production and calcium mobilization. Br. J. Pharmacol. 2007, 152, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Zhang, Y.-H.; Fang, L.-H.; Yun, Y.-P.; Lee, H.-K. Effects of tetrandrine and fangchinoline on human platelet aggregation and thromboxane B2 formation. J. Ethnopharmacol. 1999, 66, 241–246. [Google Scholar] [CrossRef]
- Coy, E.D.; Cuca, L.E.; Sefkow, M. COX, LOX and platelet aggregation inhibitory properties of Lauraceae neolignans. Bioorganic Med. Chem. Lett. 2009, 19, 6922–6925. [Google Scholar] [CrossRef]
- Abe, M.; Ozawa, Y.; Uda, Y.; Morimitsu, Y.; Nakamura, Y.; Osawa, T. A novel labdane-type trialdehyde from myoga (Zingiber mioga Roscoe) that potently inhibits human platelet aggregation and human 5-lipoxygenase. Biosci. Biotechnol. Biochem. 2006, 70, 2494–2500. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.K.; Lee, J.G.; Park, M.K.; Park, S.J.; Lee, C.H.; Park, J.H.; Kwon, S.W. Metabolomic investigation of the anti-platelet aggregation activity of ginsenoside Rk(1) reveals attenuated 12-HETE production. J. Proteome Res. 2012, 11, 4939–4946. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H. A plausible way for excretion of metal nanoparticles via active targeting. Drug Dev. Ind. Pharm. 2020, 46, 744–750. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ibrahim, M.A.; Amin, M.A.; Maswadeh, H.; Alwehaibi, M.N.; Al-Harbi, S.N.; Alharbi, Z.A.; Mohammed, H.A.; Mehany, A.B.M.; Saleem, I. Cetuximab Conjugated with Octreotide and Entrapped Calcium Alginate-beads for Targeting Somatostatin Receptors. Sci. Rep. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Dyskin, E.; Yalcin, M.; Ajayan, P.; Linhardt, R.J.; Mousa, S.A. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 2009, 20, 455104. [Google Scholar] [CrossRef]
- Kang, C.; Gwon, S.; Song, C.; Kang, P.M.; Park, S.C.; Jeon, J.; Hwang, D.W.; Lee, D. Fibrin-Targeted and H2O2-Responsive Nanoparticles as a Theranostics for Thrombosed Vessels. ACS Nano 2017, 11, 6194–6203. [Google Scholar] [CrossRef]
- Starmans, L.W.; Moonen, R.P.; Aussems-Custers, E.; Daemen, M.J.; Strijkers, G.J.; Nicolay, K.; Grull, H. Evaluation of iron oxide nanoparticle micelles for magnetic particle imaging (MPI) of thrombosis. PLoS ONE 2015, 10, e0119257. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Bachelet-Violette, L.; Rouzet, F.; Beilvert, A.; Autret, G.; Maire, M.; Menager, C.; Louedec, L.; Choqueux, C.; Saboural, P.; et al. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine 2015, 10, 73–87. [Google Scholar] [CrossRef]
- Srinivasan, R.; Marchant, R.E.; Gupta, A.S. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J. Biomed. Mater. Res. A 2010, 93, 1004–1015. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Lee, G.Y.; Kim, Y.S.; Yu, M.; Park, R.W.; Kim, I.S.; Kim, S.Y.; Byun, Y. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J. Control. Release 2006, 114, 300–306. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Sandra, F.C.; Park, D.Y.; Lee, J.Y.; Oh, K.S.; Kim, D.; Byun, Y.; Kim, I.S.; Kwon, I.C.; Kim, S.Y.; et al. Doxorubicin/heparin composite nanoparticles for caspase-activated prodrug chemotherapy. Biomaterials 2016, 101, 131–142. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Aldalaen, S.M.; Faisal, W.; Tawfeek, H.M. Somatostatin receptors as a new active targeting sites for nanoparticles. Saudi Pharm. J. 2018, 26, 1051–1059. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Liu, K.; Ma, G.; Yan, X. Co-Assembly of Heparin and Polypeptide Hybrid Nanoparticles for Biomimetic Delivery and Anti-Thrombus Therapy. Small 2016, 12, 4719–4725. [Google Scholar] [CrossRef]
- Joshi, P.; Chakraborty, S.; Dey, S.; Shanker, V.; Ansari, Z.A.; Singh, S.P.; Chakrabarti, P. Binding of chloroquine-conjugated gold nanoparticles with bovine serum albumin. J. Colloid Interface Sci. 2011, 355, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.K.; Hsu, K.H.; Cheng, Y.M.; Suen, H.Y.; Peng, S.F. Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells. Molecules 2018, 23, 3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslanka Figueroa, S.; Veser, A.; Abstiens, K.; Fleischmann, D.; Beck, S.; Goepferich, A. Influenza A virus mimetic nanoparticles trigger selective cell uptake. Proc. Natl. Acad. Sci. USA 2019, 116, 9831–9836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef]
- Janardhan, V.; Janardhan, V.; Kalousek, V. COVID-19 as a Blood Clotting Disorder Masquerading as a Respiratory Illness: A Cerebrovascular Perspective and Therapeutic Implications for Stroke Thrombectomy. J. Neuroimaging 2020, 30, 555–561. [Google Scholar] [CrossRef]
- O’Leary, K. COVID-19 vaccine and blood clotting. Nat. Med. 2021. [Google Scholar] [CrossRef]
- Saei, A.A.; Sharifi, S.; Mahmoudi, M. COVID-19: Nanomedicine Uncovers Blood-Clot Mystery. J. Proteome Res. 2020, 19, 4364–4373. [Google Scholar] [CrossRef]
- Patriota, Y.B.G.; Chaves, L.L.; Gocke, E.H.; Severino, P.; Soares, M.F.R.; Soares-Sobrinho, J.L.; Souto, E.B. Applied Nanotechnologies in Anticoagulant Therapy: From Anticoagulants to Coagulation Test Performance of Drug Delivery Systems. Appl. Nano 2021, 2, 98–117. [Google Scholar] [CrossRef]
- Song, Y.K.; Hyun, S.Y.; Kim, H.T.; Kim, C.K.; Oh, J.M. Transdermal delivery of low molecular weight heparin loaded in flexible liposomes with bioavailability enhancement: Comparison with ethosomes. J. Microencapsul. 2011, 28, 151–158. [Google Scholar] [CrossRef]
- Xue, X.; Cao, M.; Ren, L.; Qian, Y.; Chen, G. Preparation and Optimization of Rivaroxaban by Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Enhanced Oral Bioavailability and No Food Effect. AAPS PharmSciTech 2018, 19, 1847–1859. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Sapin-Minet, A.; Diab, R.; Grossiord, J.L.; Maincent, P. Low molecular weight heparin gels, based on nanoparticles, for topical delivery. Int. J. Pharm. 2012, 426, 256–262. [Google Scholar] [CrossRef]
- Bai, S.; Ahsan, F. Inhalable liposomes of low molecular weight heparin for the treatment of venous thromboembolism. J. Pharm. Sci. 2010, 99, 4554–4564. [Google Scholar] [CrossRef]
- Lavanya, N.; Muzib, Y.I.; Aukunuru, J.; Balekari, U. Preparation and evaluation of a novel oral delivery system for low molecular weight heparin. Int. J. Pharm. Investig. 2016, 6, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Matanovic, M.R.; Grabnar, P.A.; Voinovich, D.; Golob, S.; Mijovski, M.B.; Grabnar, I. Development and preclinical pharmacokinetics of a novel subcutaneous thermoresponsive system for prolonged delivery of heparin. Int. J. Pharm. 2015, 496, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Gritsch, L.; Motta, F.L.; Contessi Negrini, N.; Yahia, L.H.; Farè, S. Crosslinked gelatin hydrogels as carriers for controlled heparin release. Mater. Lett. 2018, 228, 375–378. [Google Scholar] [CrossRef]
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan based polymer-lipid hybrid nanoparticles for oral delivery of enoxaparin. Int. J. Pharm. 2018, 547, 499–505. [Google Scholar] [CrossRef]
- Tang, B.; Qian, Y.; Fang, G. Development of Lipid-Polymer Hybrid Nanoparticles for Improving Oral Absorption of Enoxaparin. Pharmaceutics 2020, 12, 607. [Google Scholar] [CrossRef]
- Ramadan, A.; Lagarce, F.; Tessier-Marteau, A.; Thomas, O.; Legras, P.; Macchi, L.; Saulnier, P.; Benoit, J.P. Oral fondaparinux: Use of lipid nanocapsules as nanocarriers and in vivo pharmacokinetic study. Int. J. Nanomed. 2011, 6, 2941–2951. [Google Scholar] [CrossRef] [Green Version]
- Eleraky, N.E.; Swarnakar, N.K.; Mohamed, D.F.; Attia, M.A.; Pauletti, G.M. Permeation-Enhancing Nanoparticle Formulation to Enable Oral Absorption of Enoxaparin. AAPS PharmSciTech 2020, 21, 88. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Agrawal, G.P.; Vyas, S.P. Biomimetic solid lipid nanoparticles for oral bioavailability enhancement of low molecular weight heparin and its lipid conjugates: In vitro and in vivo evaluation. Mol. Pharm. 2011, 8, 1314–1321. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Tawfeek, H.M.; Abdelfattah, A.; El-Saber Batiha, G.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.H.; Al-Allaf, F.A.; Bogari, N.M.; Al-Dehlawi, S.; Qari, S.H. Strategies for Vaccination: Conventional Vaccine Approaches Versus New-Generation Strategies in Combination with Adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef]
- WHO Issues Emergency Use Listing to Novavax-Serum Institute’s COVID-19 Vaccine. Reuters. Available online: https://www.channelnewsasia.com/asia/novavax-serum-institute-covovax-covid19-vaccine-who-emergency-use-listing-2387491 (accessed on 27 November 2021).
- Henderson, J. What Happened to the Novavax Vaccine? Medpage Today. 2022. Available online: https://www.medpagetoday.com/special-reports/exclusives/96461 (accessed on 27 November 2021).
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Medhi, R.; Srinoi, P.; Ngo, N.; Tran, H.-V.; Lee, T.R. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl. Nano Mater. 2020, 3, 8557–8580. [Google Scholar] [CrossRef]
- Zimmer, C. Researchers Are Hatching a Low-Cost Coronavirus Vaccine. The New York Times, 5 April 2021; ISSN 0362-4331. [Google Scholar]
- Wadman, M. The long shot. Science 2020, 370, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Wadman, M. Novavax launches pivotal U.S. trial of dark horse COVID-19 vaccine after manufacturing delays. Science 2020. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Potzsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie 2021, 41, 184–189. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Scobie, H.; Wallace, M.; Hadler, S.C.; Leung, J.; Blain, A.E.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine—United States, February 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 329–332. [Google Scholar] [CrossRef]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 651. [Google Scholar]
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef]
- Billy, E.; Clarot, F.; Depagne, C.; Korsia-Meffre, S.; Rochoy, M.; Zores, F. Thrombotic events after AstraZeneca vaccine: What if it was related to dysfunctional immune response? Therapie 2021, 76, 367–369. [Google Scholar] [CrossRef]
| Drugs | Types of Nanoparticles | Size Range (nm) | References |
|---|---|---|---|
| Low-molecular-weight heparin (LMWH) | Liposomes | 80–90 | [159] |
| Ardeparin (LMWH) | 100–150 | [162] | |
| Enoxaparin (LMWH) | 40–65 | [163] | |
| Unfractionated (UFH) heparin | Nanogel | 130 | [164] |
| Bemiparin (LMWH) Nadroparin (LMWH) Tinzaparin (LMWH) | 150–400 | [161] | |
| Enoxaparin (LMWH) | 100–1000 | [165] | |
| Enoxaparin | Polymeric nanoparticles | 280–320 | [166,167] |
| Fondaparinux | 40–65 | [168] | |
| Enoxaparin | 180–195 | [169] | |
| (LMWH) | Solid lipid nanoparticles | 280–380 | [170] |
| Enoxaparin (LMWH) | Self-nanoemulsifying drug delivery system | 30–245 | [167] |
| Rivaroxaban (Factor Xa inhibitor) | 50–150 | [160] |
| Characteristics | Pfizer/BioNTech | Oxford University/AstraZeneca | Moderna | Nuvaxovid and Covovax |
|---|---|---|---|---|
| Therapeutic indication | For effective immunization to suppress SARS-CoV-2 virus-induced COVID-19 in persons 16 years of age and over. | For effective immunization for the prevention of COVID-19 in persons 18 years of age and over. | For effective immunization to prevent SARS-CoV-2 virus-induced COVID-19 in persons 18 years of age and over. | The vaccine is administered in two doses and is stable at refrigerated temperatures of 2 to 8 °C (36 to 46 °F). |
| Type of vaccine | Messenger RNA (mRNA) | Adenovirus vector | Messenger RNA (mRNA) | Recombinant nanoparticle vaccine |
| Number of doses | A multidose vial | One dose | Multidose | Multidose |
| Pharmaceutical form | Concentrate for solution for injection. | Solution for injection. | Dispersion for injection. | Dispersion for injection. |
| Dosage schedule | Two doses (0.3 mL each) with an interval of between 3 to 12 weeks. | Two doses (0.5 mL each) with an interval of between 4 and 12 weeks. | Two doses (0.5 mL each). It is recommended that the second dose be administered 28 days after the first dose. | The vaccine requires two doses and is stable at 2 to 8 °C (36 to 46 °F) refrigerated temperatures. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Bakky, M.S.; Amin, E.; Ewees, M.G.; Mahmoud, N.I.; Mohammed, H.A.; Altowayan, W.M.; Abdellatif, A.A.H. Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines. Viruses 2022, 14, 228. https://doi.org/10.3390/v14020228
Abdel-Bakky MS, Amin E, Ewees MG, Mahmoud NI, Mohammed HA, Altowayan WM, Abdellatif AAH. Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines. Viruses. 2022; 14(2):228. https://doi.org/10.3390/v14020228
Chicago/Turabian StyleAbdel-Bakky, Mohamed S., Elham Amin, Mohamed G. Ewees, Nesreen I. Mahmoud, Hamdoon A. Mohammed, Waleed M. Altowayan, and Ahmed A. H. Abdellatif. 2022. "Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines" Viruses 14, no. 2: 228. https://doi.org/10.3390/v14020228
APA StyleAbdel-Bakky, M. S., Amin, E., Ewees, M. G., Mahmoud, N. I., Mohammed, H. A., Altowayan, W. M., & Abdellatif, A. A. H. (2022). Coagulation System Activation for Targeting of COVID-19: Insights into Anticoagulants, Vaccine-Loaded Nanoparticles, and Hypercoagulability in COVID-19 Vaccines. Viruses, 14(2), 228. https://doi.org/10.3390/v14020228









