Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. NADES Preparation
2.4. Preliminary Study Design and Extraction Procedures
2.5. Box–Behnken Design (BBD) Optimization
2.6. Extracts Characterization
2.6.1. Total Polyphenol Content (TPC)
2.6.2. Free DPPH• Scavenging Activity Assay
2.6.3. Determination of Ellagic Acid Content by HPLC/DAD
2.6.4. Nuclear Magnetic Resonance (NMR)
2.6.5. Anti-Proliferative Activity and Cytotoxicity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Study
3.2. Box–Behnken Design (BBD) and Response Surface Methodology Optimization
3.2.1. Model Adequacy
3.2.2. Effect of Independent Variables on Investigated Responses
3.2.3. Multi-Response Optimization
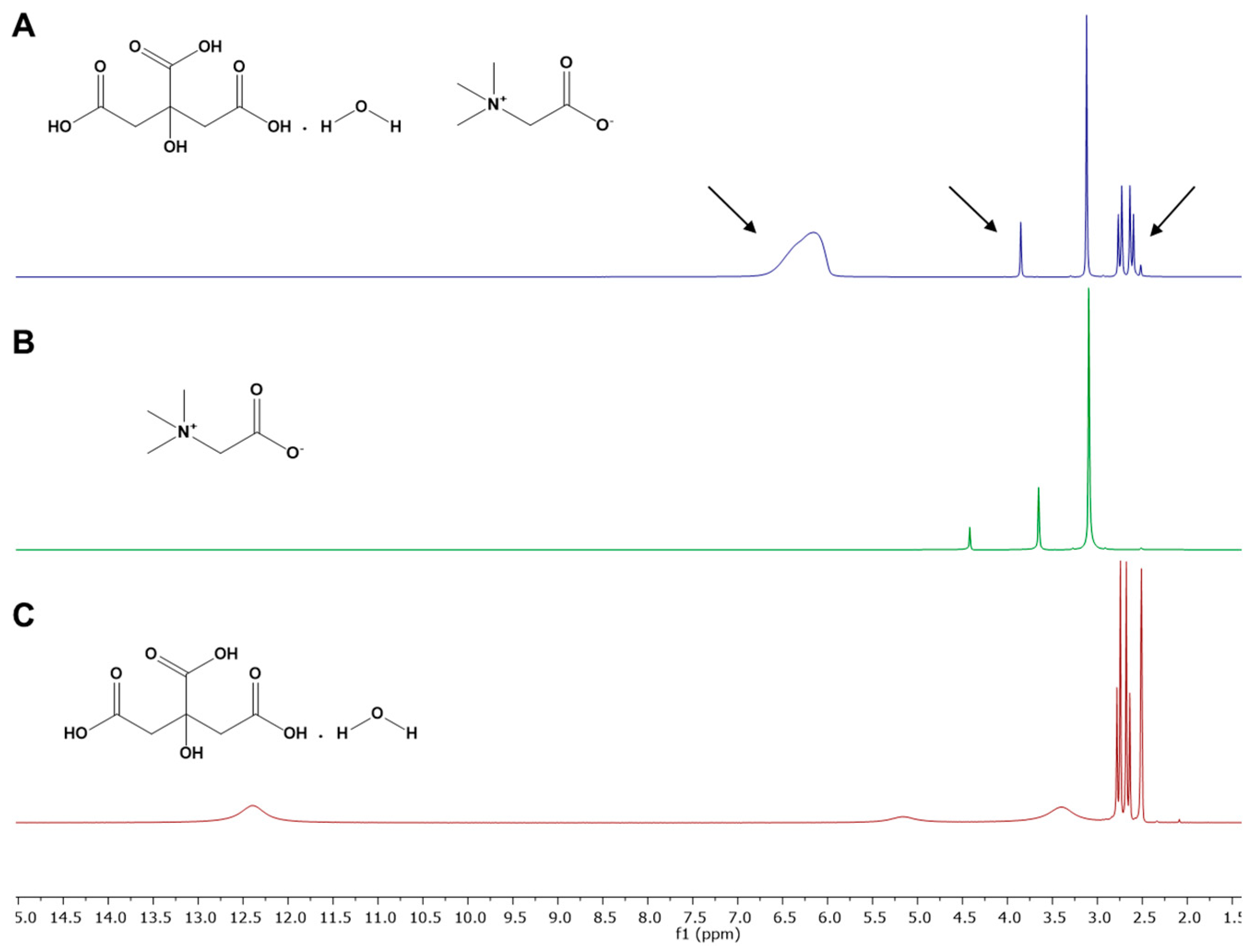
3.3. NMR
3.4. Anti-Proliferative Activity and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. World Production of Raspberries; FAOSTAT: Rome, Italy, 2021. [Google Scholar]
- Marić, B.; Abramović, B.; Ilić, N.; Krulj, J.; Kojić, J.; Perović, J.; Bodroža-Solarov, M.; Teslić, N. Valorization of red raspberry (Rubus idaeus L.) seeds as a source of health beneficial compounds: Extraction by different methods. J. Food Process. Preserv. 2020, 44, e14744. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J.; Yang, J.; Hao, Y.; Fan, Y.; Wang, C.; Li, N. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT-Food Sci. Technol. 2021, 135, 109995. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Grilli, M.; Pittaluga, A.M.; Boggia, R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur. J. Med. Chem. 2019, 183, 111724. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kucharczyk, E.; Kaliszan, R.; Markuszewski, M.; Fotschki, B.; Juśkiewicz, J.; Borkowska-Sztachańska, M.; Ognik, K. The characterization of ground raspberry seeds and the physiological response to supplementation in hypertensive and normotensive rats. Nutrients 2020, 12, 1630. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Lim, S.C.; Hwang, H.; Han, S.I. Ellagic acid inhibits extracellular acidity-induced invasiveness and expression of COX1, COX2, snail, twist 1, and c-myc in gastric carcinoma cells. Nutrients 2019, 11, 3023. [Google Scholar] [CrossRef] [Green Version]
- Yousef, A.I.; El-Masry, O.S.; Yassin, E.H. The anti-oncogenic influence of ellagic acid on colon cancer cells in leptin-enriched microenvironment. Tumor Biol. 2016, 37, 13345–13353. [Google Scholar] [CrossRef]
- Theocharis, G.; Andlauer, W. Innovative microwave-assisted hydrolysis of ellagitannins and quantification as ellagic acid equivalents. Food Chem. 2013, 138, 2430–2434. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Abdullah, M.F.; Sam, S.T. Hydrolysis empty fruit bunch (EFB) using green solvent. In Proceedings of the International Conference on Advanced Manufacturing and Industry Applications, Sarawak, Malaysia, 15–17 August 2018; IOP Conference Series: Materials Science and Engineering. Volume 429, p. 012059. [Google Scholar]
- Mamilla, J.L.; Novak, U.; Grilc, M.; Likozar, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Kojić, J.; Krulj, J.; Ilić, N.; Lončar, E.; Pezo, L.; Mandić, A.; Bodroža Solarov, M. Analysis of betaine levels in cereals, pseudocereals and their products. J. Funct. Foods. 2017, 37, 157–163. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Nawani, N.N.; Kapadnis, B.P. Optimization of chitinase production using statistics based experimental designs. Process Biochem. 2005, 40, 651–660. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Boyacioglu, D.; Capanoglu, E. Optimization of extraction of bioactive compounds from black carrot using response surface methodology (RSM). Food Anal. Methods 2016, 9, 1876–1886. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Pavlić, B.; Pezo, L.; Marić, B.; Tukuljac, L.P.; Zeković, Z.; Solarov, M.B.; Teslić, N. Supercritical fluid extraction of raspberry seed oil: Experiments and modelling. J. Supercrit. Fluids 2020, 157, 104687. [Google Scholar] [CrossRef]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Ilić, N.; Teslić, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT-Food Sci. Technol. 2020, 130, 109627. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Meneses, L.; Santos, F.; Gameiro, A.R.; Paiva, A.; Duarte, A.R.C. Preparation of binary and ternary deep eutectic systems. J. Vis. Exp. 2019, e60326. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Oliveira, F.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C. Untangling the bioactive properties of therapeutic deep eutectic solvents based on natural terpenes. Curr. Res. Chem. Biol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- An, J.Y.; Wang, L.T.; Lv, M.J.; Wang, J.D.; Cai, Z.H.; Wang, Y.Q.; Zhang, S.; Yang, Q.; Fu, Y.J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Ren, S.H.; Xiao, Y.; Wang, Y.M.; Kong, J.; Hou, Y.C.; Wu, W.Z. Effect of water on the separation of phenol from model oil with choline chloride via forming deep eutectic solvent. Fuel Process. Technol. 2015, 137, 104–108. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Bursać Kovačević, D.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and conventional valorizations of grape seeds from winery by-products as sustainable source of lipophilic antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Suuronen, J.; Pitkänen, I.; Halttunen, H.; Moilanen, R. Formation of the main gas compounds during thermal analysis and pyrolysis. Betaine and betaine monohydrate. J. Therm. Anal. Calorim. 2002, 69, 359–369. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Bera, O.; Oktem Olgun, E.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Process. 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef]
- Losso, J.N.; Bansode, R.R.; Trappey, A., II; Bawadi, H.A.; Truax, R. In vitro anti-proliferative activities of ellagic acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.M.; Popa, G.; Gill, C.I.R.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.; Li, X.Q.; Weber, C.; Lee Young, C.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]

| Sample | TP [mg GAE 1/g DW] | DPPH [μM TE 2/g DW 3] | Ellagic Acid [mg/100 g DW] | Ellagic Acid [mg/100 g Extract] |
|---|---|---|---|---|
| LA:FRC:H2O (5:1:0.8) | 39.80 ± 0.80 h | 412.15 ± 26.15 c,d | 63.86 ± 2.13 a,b | 2.70 ± 0.08 b |
| LA:GLC:H2O (5:1:0.8) | 31.72 ± 0.90 d | 406.91 ± 6.57 c,d | 66.18 ± 1.66 a,b | 2.76 ± 0.05 b |
| MA:BET (1:1) | 33.27 ± 1.43 d,e | 375.84 ± 8.34 b,c | 61.78 ± 3.71 a,b | 2.95 ± 0.36 b |
| MA:BET (2:1) | 34.07 ± 0.73 d,e,f | 406.36 ± 10.24 c,d | 72.01 ± 2.87 b | 3.01 ± 0.07 b |
| TAR:BET (1:1) | 26.91 ± 0.64 b,c | 348.14 ± 16.60 b | 60.13 ± 1.81 a,b | 2.59 ± 0.26 b |
| TAR:BET (2:1) | 30.49 ± 1.82 c,d | 352.34 ± 27.48 b | 71.23 ± 2.11 b | 3.18 ± 0.22 b |
| LA:BET:H2O (1:1:0.16) | 39.02 ± 1.69 g,h | 410.23 ± 9.21 c,d | 63.53 ± 1.82 a,b | 3.18 ± 0.56 b |
| LA:BET:H2O (2:1:0.32) | 35.43 ± 0.61 e,f,g | 431.18 ± 6.62 d | 63.43 ± 0.98 a,b | 3.05 ± 0.38 b |
| CA:BET:H2O (1:1:1) | 31.23 ± 2.59 d | 387.15 ± 13.46 b,c | 66.07 ± 4.31 a,b | 2.80 ± 0.15 b |
| CA:BET:H2O (2:1:2) | 32.57 ± 1.45 d,e | 401.20 ± 8.45 c,d | 75.17 ± 3.14 b | 3.20 ± 0.13 b |
| ETOH 80% | 20.67 ± 0.73 a | 197.78 ± 2.76 a | 45.23 ± 0.88 a | 1.37 ± 0.03 a |
| 2M HCl in MEOH 4 | 37.33 ± 1.46 f,g,h | 4128.92 ± 35.91 f | 1031.41 ± 20.73 d | 4.34 ± 0.09 c |
| 2M HCl in MEOH | 26.54 ± 2.30 b | 597.10 ± 8.38 e | 703.28 ± 16.25 c | 20.82 ± 0.48 d |
| Sample | A: Temperature [°C] | B: Moles of Citric Acid Monohydrate | C: Water Content [g/100 g] | D: Time [min] | E: NADES/Plant Ratio [g/g] | TP [mg GAE 1/g DW] | DPPH [μM TE 2/g DW 3] | Ellagic Acid [mg/100 g DW] | Ellagic Acid [mg/100 g Extract] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid/liquid extraction | ||||||||||||||
| 1 | −1 | 60 | 1 | 3 | 1 | 25 | 1 | 60 | −1 | 10 | 34.01 ± 1.05 a,b,c,d | 650.43 ± 4.28 c,d,e | 45.66 ± 0.24 a,b | 3.45 ± 0.09 d |
| 2 | 1 | 70 | 1 | 3 | −1 | 20 | 1 | 60 | −1 | 10 | 33.25 ± 2.60 a,b,c,d | 637.13 ± 12.26 c | 52.56 ± 0.03 c,d | 3.91 ± 0.01 f |
| 3 | 1 | 70 | 1 | 3 | 1 | 25 | 1 | 60 | 1 | 20 | 37.21 ± 0.32 e,f | 427.99 ± 9.87 a | 72.96 ± 0.90 f | 2.74 ± 0.05 c |
| 4 | 1 | 70 | 1 | 3 | 1 | 25 | −1 | 30 | −1 | 10 | 35.15 ± 1.33 b,c,d,e,f | 679.23 ± 10.31 d,e | 49.30 ± 0.17 b,c | 3.64 ± 0.01 e |
| 5 | 1 | 70 | −1 | 2 | 1 | 25 | −1 | 30 | 1 | 20 | 36.62 ± 1.41 d,e,f | 454.28 ± 11.16 a,b | 65.33 ± 0.89 e | 2.50 ± 0.04 a,b |
| 6 | −1 | 60 | 1 | 3 | −1 | 20 | −1 | 30 | −1 | 10 | 33.70 ± 0.43 a,b,c,d,e | 632.19 ± 11.41 c | 45.25 ± 1.79 a | 3.39 ± 0.09 d |
| 7 | 1 | 70 | −1 | 2 | −1 | 20 | 1 | 60 | 1 | 20 | 35.21 ± 0.93 b,c,d,e,f | 447.31 ± 2.09 a,b | 70.67 ± 0.94 f | 2.64 ± 0.02 b,c |
| 8 | −1 | 60 | 1 | 3 | −1 | 20 | 1 | 60 | 1 | 20 | 31.48 ± 0.80 a | 480.17 ± 17.37 b | 62.56 ± 0.71 e | 2.41 ± 0.01 a |
| 9 | −1 | 60 | −1 | 2 | −1 | 20 | 1 | 60 | −1 | 10 | 32.20 ± 0.87 a,b | 643.54 ± 2.12 c,d | 45.00 ± 0.19 a | 3.42 ± 0.01 d |
| 10 | 1 | 70 | 1 | 3 | −1 | 20 | −1 | 30 | 1 | 20 | 32.77 ± 0.28 a,b,c | 451.86 ± 14.34 a,b | 64.18 ± 0.95 e | 2.43 ± 0.03 a |
| 11 | −1 | 60 | −1 | 2 | 1 | 25 | −1 | 30 | −1 | 10 | 36.05 ± 0.66 c,d,e,f | 655.80 ± 3.56 c,d,e | 47.33 ± 2.17 a,b | 3.52 ± 0.14 d,e |
| 12 | 1 | 70 | −1 | 2 | −1 | 20 | −1 | 30 | −1 | 10 | 32.74 ± 1.63 a,b,c | 641.05 ± 12.33 c | 45.35 ± 0.78 a,b | 3.43 ± 0.01 d |
| 13 | −1 | 60 | −1 | 2 | 1 | 25 | 1 | 60 | 1 | 20 | 36.29 ± 0.10 c,d,e,f | 455.17 ± 3.03 a,b | 69.43 ± 3.87 f | 2.56 ± 0.09 a,b |
| 14 | −1 | 60 | −1 | 2 | −1 | 20 | −1 | 30 | 1 | 20 | 33.84 ± 0.44 a,b,c,d,e | 436.22 ± 11.59 a | 64.71 ± 0.47 e | 2.46 ± 0.03 a |
| 15 | 1 | 70 | −1 | 2 | 1 | 25 | 1 | 60 | −1 | 10 | 38.72 ± 1.90 f | 682.23 ± 29.08 e | 53.50 ± 0.14 d | 3.94 ± 0.03 f |
| 16 | −1 | 60 | 1 | 3 | 1 | 25 | −1 | 30 | 1 | 20 | 35.08 ± 1.19 b,c,d,e | 474.21 ± 14.51 b | 65.24 ± 0.29 e | 2.45 ± 0.02 a |
| Additional experiments with ultrasound-assisted extraction | ||||||||||||||
| 17 | 1 | 70 | 1 | 3 | −1 | 20 | 1 | 60 | −1 | 10 | nd | nd | 47.58 ± 0.23 | 2.06 ± 0.02 |
| 18 | 1 | 70 | −1 | 2 | 1 | 25 | 1 | 60 | −1 | 10 | nd | nd | 51.30 ± 2.58 | 2.11 ± 0.11 |
| Sample | A: Temperature [°C] | B: Time [min] | C: NADES/Plant Ratio [g/g] | TP [mg GAE 1/g DW 2] | DPPH [μM TE 3/g DW] | Ellagic Acid [mg/100 g DW] | Ellagic Acid [mg/100 g Extract] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | 65 | 0 | 100 | −1 | 15 | 35.54 ± 1.50 a | 1051.71 ± 19.65 c,d | 65.38 ± 2.15 a | 3.19 ± 0.03 e,f |
| 2 | −1 | 65 | −1 | 50 | 0 | 25 | 41.27 ± 0.99 c,d | 601.10 ± 16.24 a | 85.14 ± 7.83 b,c | 2.47 ± 0.15 b,c |
| 3 | 0 | 75 | 1 | 150 | −1 | 15 | 37.99 ± 0.92 a,b | 1087.24 ± 52.50 d | 83.56 ± 3.61 b | 4.04 ± 0.13 g |
| 4 | 1 | 85 | 1 | 150 | 0 | 25 | 43.85 ± 1.23 d,e,f | 582.99 ± 21.23 a | 143.07 ± 5.00 f | 4.02 ± 0.12 g |
| 5 | 0 | 75 | −1 | 50 | −1 | 15 | 36.30 ± 0.85 a,b | 1004.22 ± 51.82 c | 67.09 ± 1.35 a | 3.31 ± 0.04 f |
| 6 | 1 | 85 | −1 | 50 | 0 | 25 | 42.04 ± 0.40 c,d,e | 634.85 ± 7.96 a | 104.16 ± 7.25 e | 2.99 ± 0.16 d,e |
| 7 | 1 | 85 | 0 | 100 | 1 | 35 | 45.76 ± 0.82 f | 760.91 ± 30.64 b | 151.56 ± 6.04 f | 2.97 ± 0.08 d,e |
| 8 | 0 | 75 | 1 | 150 | 1 | 35 | 43.97 ± 1.90 d,e,f | 809.30 ± 20.89 b | 137.62 ± 4.39 f | 2.68 ± 0.05 c,d |
| 9 | 0 | 75 | 0 | 50 | 0 | 25 | 43.46 ± 1.11 d,e,f | 617.87 ± 29.22 a | 105.93 ± 5.39 e | 3.03 ± 0.10 e,f |
| 10 | −1 | 65 | 0 | 50 | 1 | 35 | 43.38 ± 0.88 d,e,f | 820.22 ± 5.32 b | 102.97 ± 4.10 d,e | 2.12 ± 0.07 a |
| 11 | 0 | 75 | 0 | 50 | 0 | 25 | 42.61 ± 1.52 d,e,f | 597.14 ± 1.43 a | 101.09 ± 4.26 d,e | 2.96 ± 0.06 d,e |
| 12 | −1 | 65 | 1 | 150 | 0 | 25 | 42.59 ± 0.58 d,e,f | 621.45 ± 9.81 a | 88.06 ± 6.75 b,c,d | 2.61 ± 0.12 c |
| 13 | 0 | 75 | 0 | 100 | 0 | 25 | 43.83 ± 0.89 d,e,f | 627.10 ± 7.10 a | 101.84 ± 4.99 d,e | 2.97 ± 0.10 d,e |
| 14 | 1 | 85 | 0 | 100 | −1 | 15 | 38.85 ± 0.73 b,c | 1257.69 ± 32.32 e | 99.40 ± 4.66 c,d,e | 4.83 ± 0.17 h |
| 15 | 0 | 75 | −1 | 50 | 1 | 35 | 44.91 ± 0.52 e,f | 800.80 ± 5.17 b | 109.74 ± 3.62 e | 2.22 ± 0.04 a,b |
| Response | Equation |
|---|---|
| TP | |
| DPPH | |
| Ellagic acid | |
| Ellagic acid extract |
| Sample | A: Temperature [°C] | B: Time [min] | C: Solvent/Plant Ratio [g/g] | TP [mg GAE 1/g DW 2] | DPPH [μM TE 3/g DW] | Ellagic Acid [mg/100 g DW] | Ellagic Acid [mg/100 g Extract] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Predicted responses | ||||||||||
| Opt1-pred | 1 | 85 | 0.01 | 100.45 | 1 | 35 | 45.28 | 772.59 | 151.56 | 2.96 |
| Opt2-pred | 1 | 85 | 0.94 | 147.00 | −0.92 | 15.76 | 39.69 | 1125.64 | 114.48 | 5.07 |
| Experimentally obtained responses | ||||||||||
| Opt1 | 1 | 85 | 0.01 | 100.45 | 1 | 35 | 44.77 ± 1.76 c | 707.25 ± 2.87 b | 147.02 ± 7.18 c | 2.98 ± 0.13 b |
| Opt2 | 1 | 85 | 0.94 | 147.00 | −0.92 | 15.76 | 40.16 ± 1.90 b | 995.18 ± 32.08 c | 117.94 ± 5.93 b | 5.21 ± 0.17 d |
| Opt3 * | 95 | 240 | 10 | 37.97 ± 0.60 b | 679.97 ± 27.45 b | 115.62 ± 4.41 b | 7.93 ± 0.15 e | |||
| ETOH 80% | 60 | 60 | 20 | 20.67 ± 0.73 a | 197.78 ± 2.76 a | 45.23 ± 0.88 a | 1.37 ± 0.03 a | |||
| 2M HCl in MEOH | 85 | 150 | 170 | 37.33 ± 1.46 b | 4128 ± 35.91 d | 1031.41 ± 20.73 d | 4.34 ± 0.09 c | |||
| Systems/Compounds | EC50 (mg/mL) | Selectivity Index | |
|---|---|---|---|
| Cytotoxicity (Caco-2) | Anti-proliferative Activity (HT29) | ||
| Opt3—1 a | 3.80 ± 3.33 | 3.18 ± 0.37 | 1.20 |
| Opt3—2 a | ≈4.63 * | 2.16 ± 0.49 | 2.14 |
| Opt3—3 a | 4.60 ± 2.80 | ≈3.84 * | 1.20 |
| ETOH 80%—1 b | 103.10 ± 7.04 | 48.65 ± 11.54 | 2.12 |
| ETOH 80%—2 b | ≈120.60 * | 22.93 ± 4.41 | 5.26 |
| ETOH 80%—3 b | 115.30 ± 12.25 | 61.82 ± 3.17 | 1.87 |
| CA:BET:H2O (3:1:3) | 8.06 ± 4.26 | 2.08 ± 0.14 | 3.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teslić, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojić, M.; Mandić, A.; Pavlić, B.; Kljakić, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. https://doi.org/10.3390/antiox11020254
Teslić N, Santos F, Oliveira F, Stupar A, Pojić M, Mandić A, Pavlić B, Kljakić AC, Duarte ARC, Paiva A, et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants. 2022; 11(2):254. https://doi.org/10.3390/antiox11020254
Chicago/Turabian StyleTeslić, Nemanja, Filipa Santos, Filipe Oliveira, Alena Stupar, Milica Pojić, Anamarija Mandić, Branimir Pavlić, Aleksandra Cvetanović Kljakić, Ana Rita C. Duarte, Alexandre Paiva, and et al. 2022. "Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES)" Antioxidants 11, no. 2: 254. https://doi.org/10.3390/antiox11020254
APA StyleTeslić, N., Santos, F., Oliveira, F., Stupar, A., Pojić, M., Mandić, A., Pavlić, B., Kljakić, A. C., Duarte, A. R. C., Paiva, A., & Mišan, A. (2022). Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants, 11(2), 254. https://doi.org/10.3390/antiox11020254












