Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers
Abstract
:1. Introduction
2. Results and Discussion
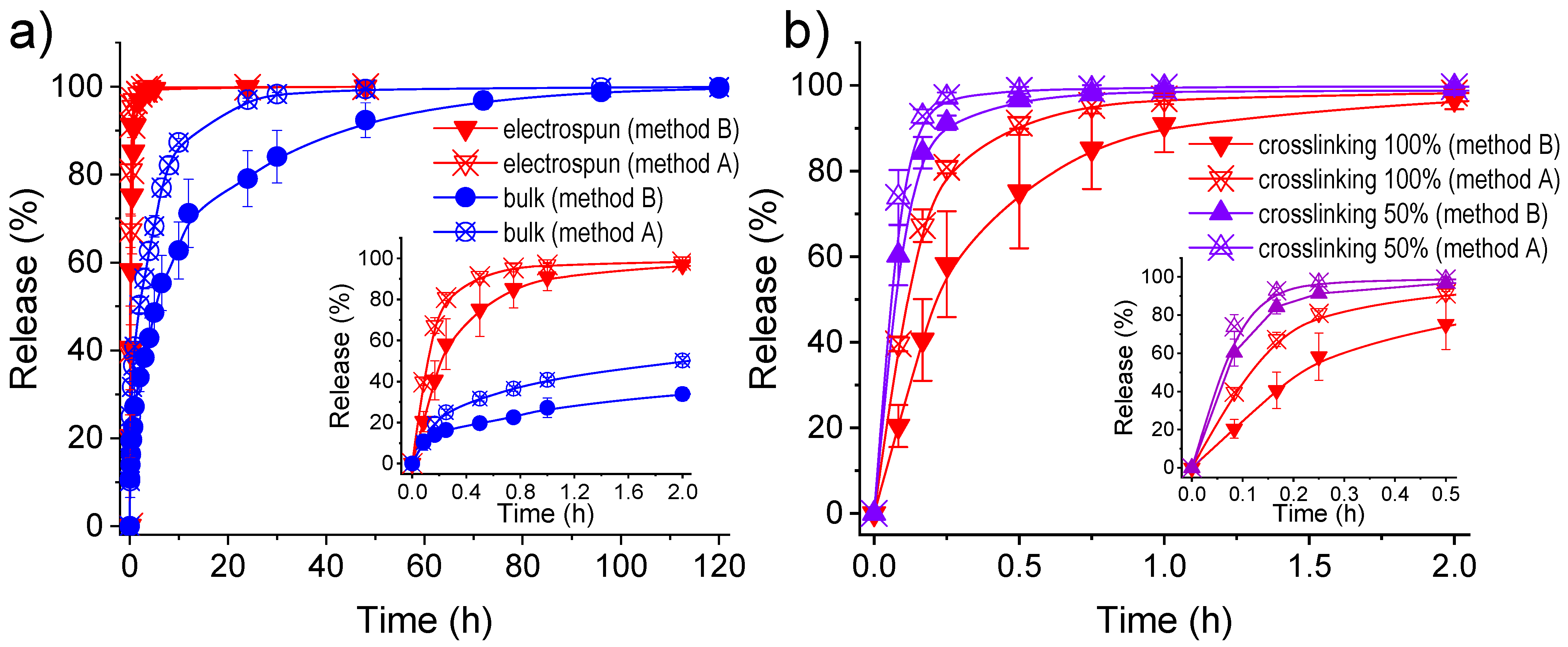
2.1. Electrospinning of γ-PGA
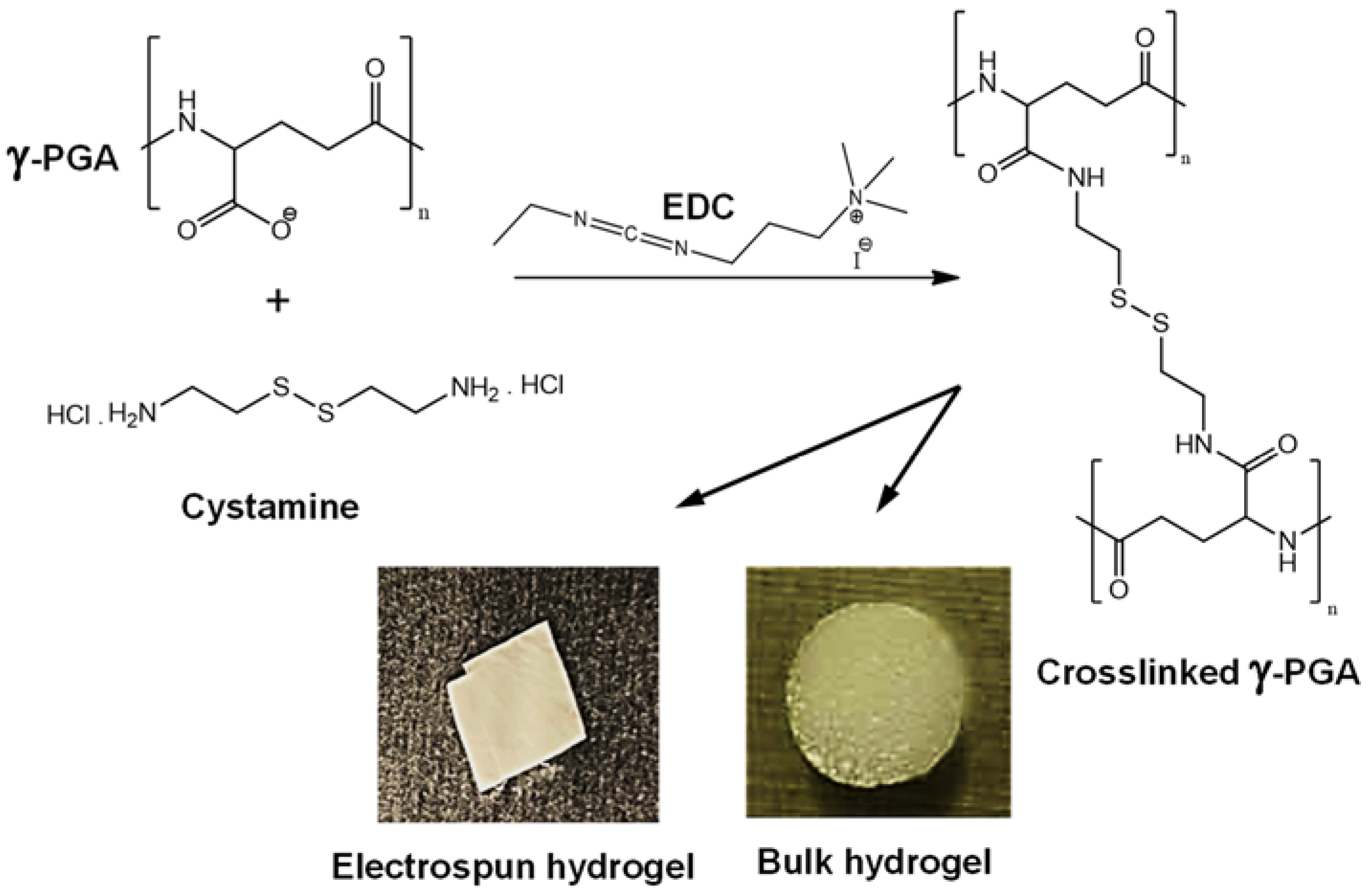
2.2. Preparation of γ-PGA Hydrogels
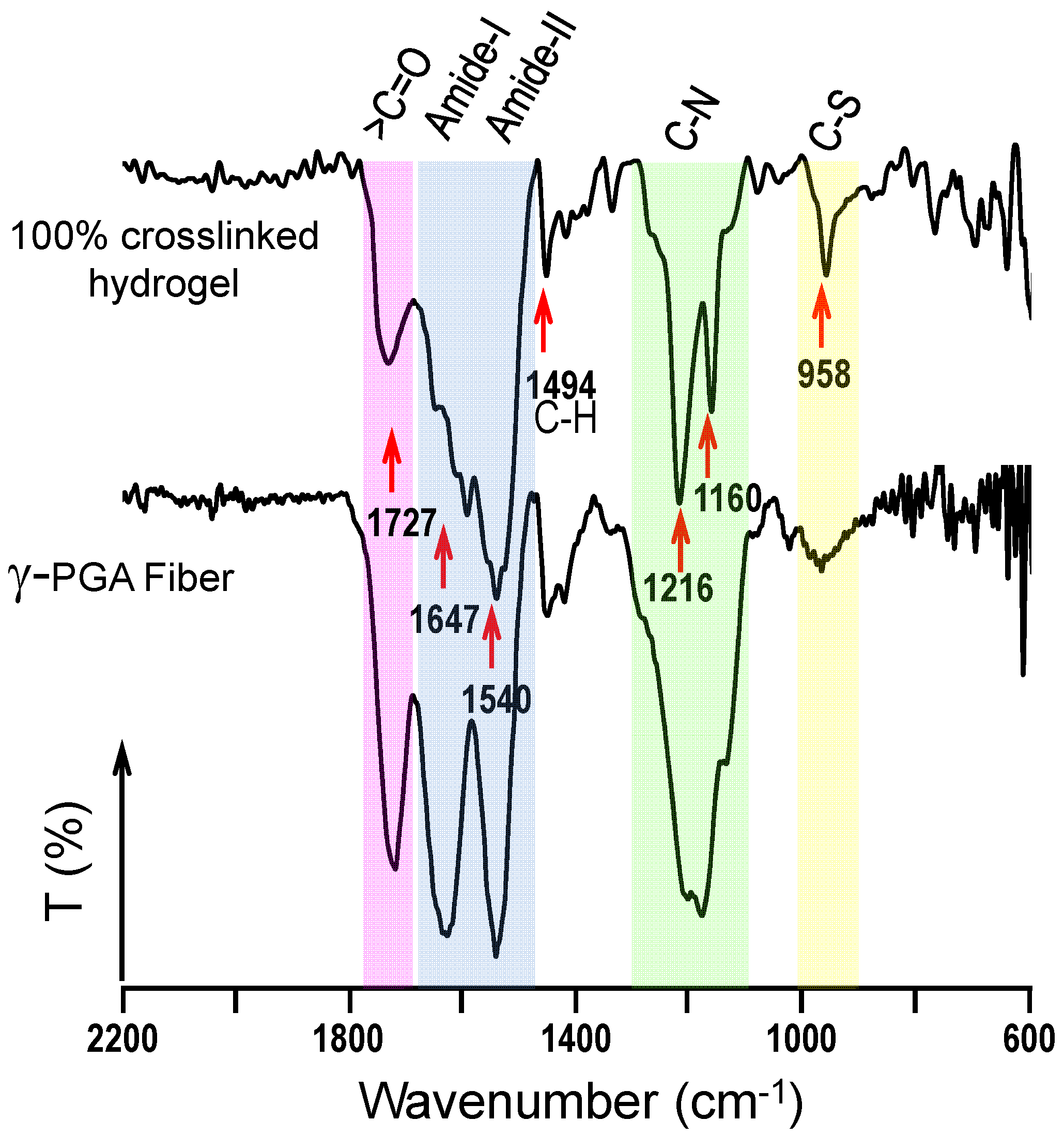
2.3. Physical Characterization of Hydrogels
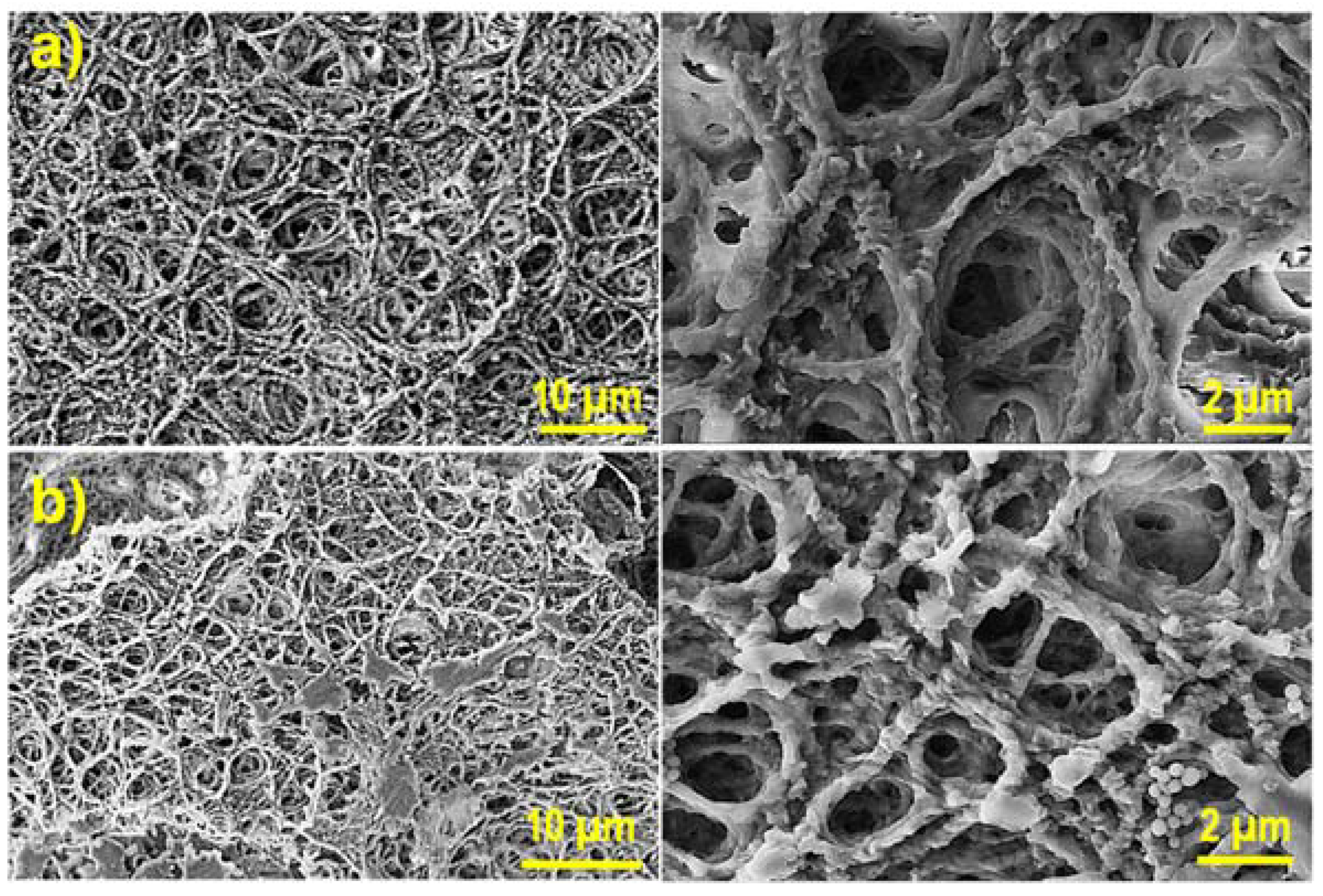
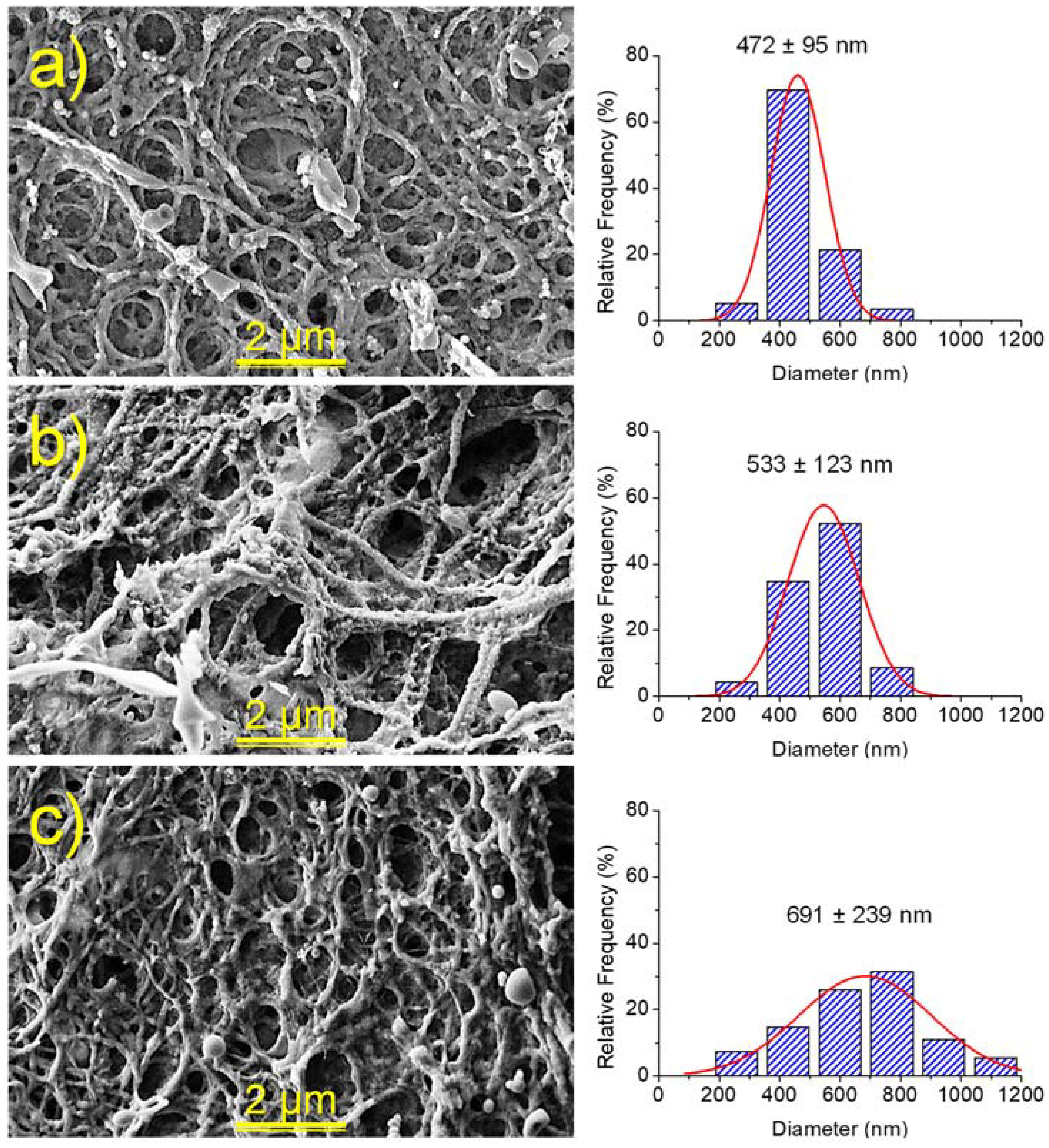
2.4. Morphology of Hydrogels
2.5. γ-PGA Hydrogels for the Release of Triclosan as a Hydrophobic Drug
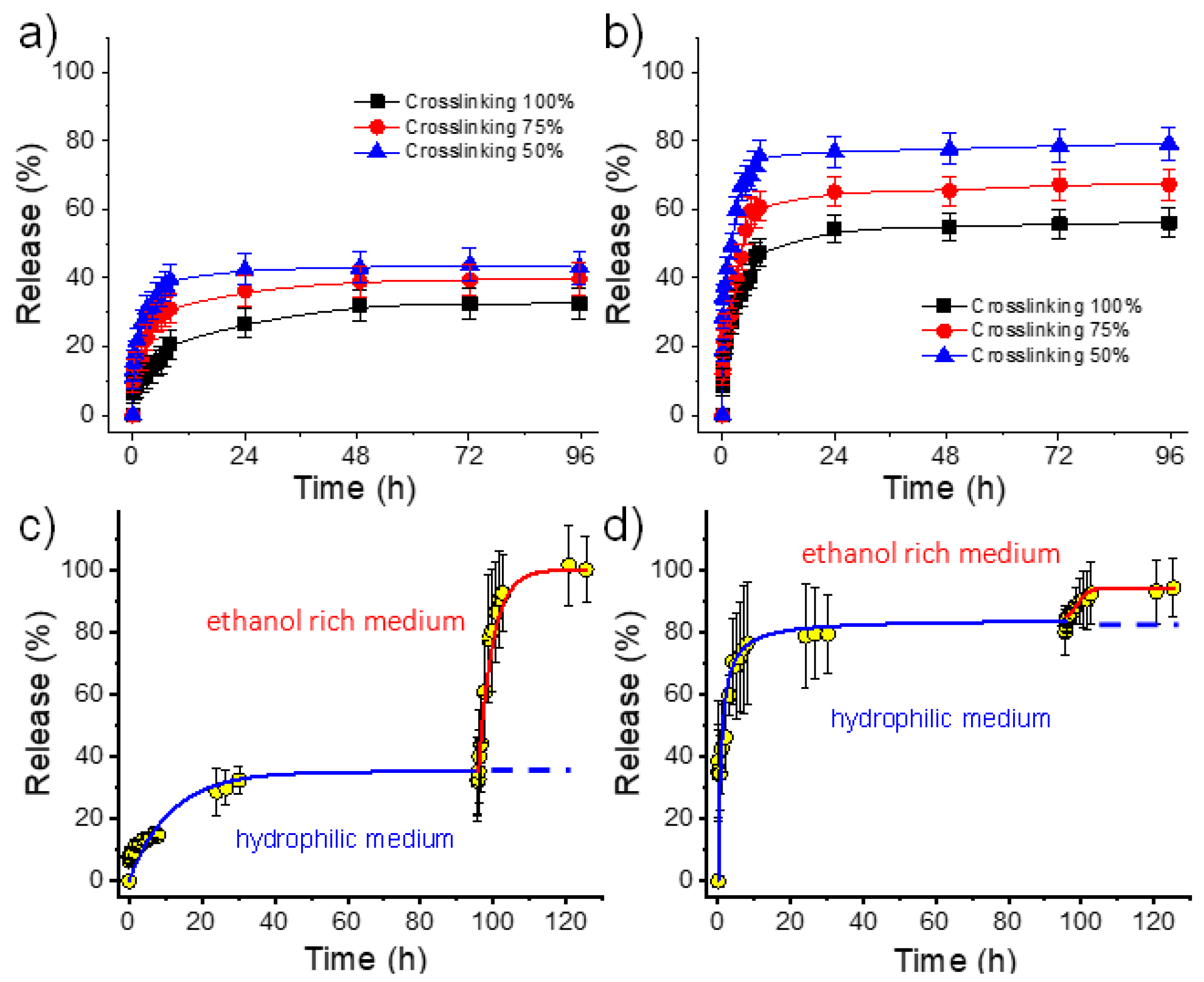
2.6. γ-PGA Hydrogels for the Release of Chlorhexidine and Polyguanide as Hydrophilic Drugs
- (1)
- Both hydrophilic drugs can be released to the hydrophilic PBS medium in contrast with the virtual zero release of the hydrophobic TCS.
- (2)
- PHMB has a greater burst effect than CHX and has also a higher release rate (Table 3, and Figure 10). Differences are highly significant and indicate a clearly higher difficulty of the larger PHMB molecules to be encapsulated into the inner parts of the hydrogel, and even higher affinity with the PBS release medium due to its higher hydrophilicity. In fact, encapsulation capacities (EC, Equation (2)) around 0.07% and 0.78% were determined for CHX and PHMB, respectively, for the 100% crosslinked hydrogels. Note also that highly different saturation levels were reached during the release. Specifically, values of 25% and 52% were found after 96 h of exposure for CHX and PHMB loaded samples with the higher theoretical crosslinking degree, respectively.
- (3)
- In all cases, the release rate was slightly dependent on the theoretical crosslinking degree and logically decreased when it increased (Table 3).
- (4)
- A practically complete release was found for both drugs using a PBS/ethanol mixture since saturation problems could be avoided. In this case, the release rate was found to be slightly higher for CHX. Figure 10c,d clearly demonstrated that the retained drug after PBS exposure could be completely released by a simple change of the medium. Specifically, after 24–96 h of exposure to PBS, the release increased from 40% to 100% for CHX and from 80% to 92% for PHMB when ethanol was added to the medium.
2.7. Antibacterial Activity of γ-PGA Hydrogels Loaded with Drugs
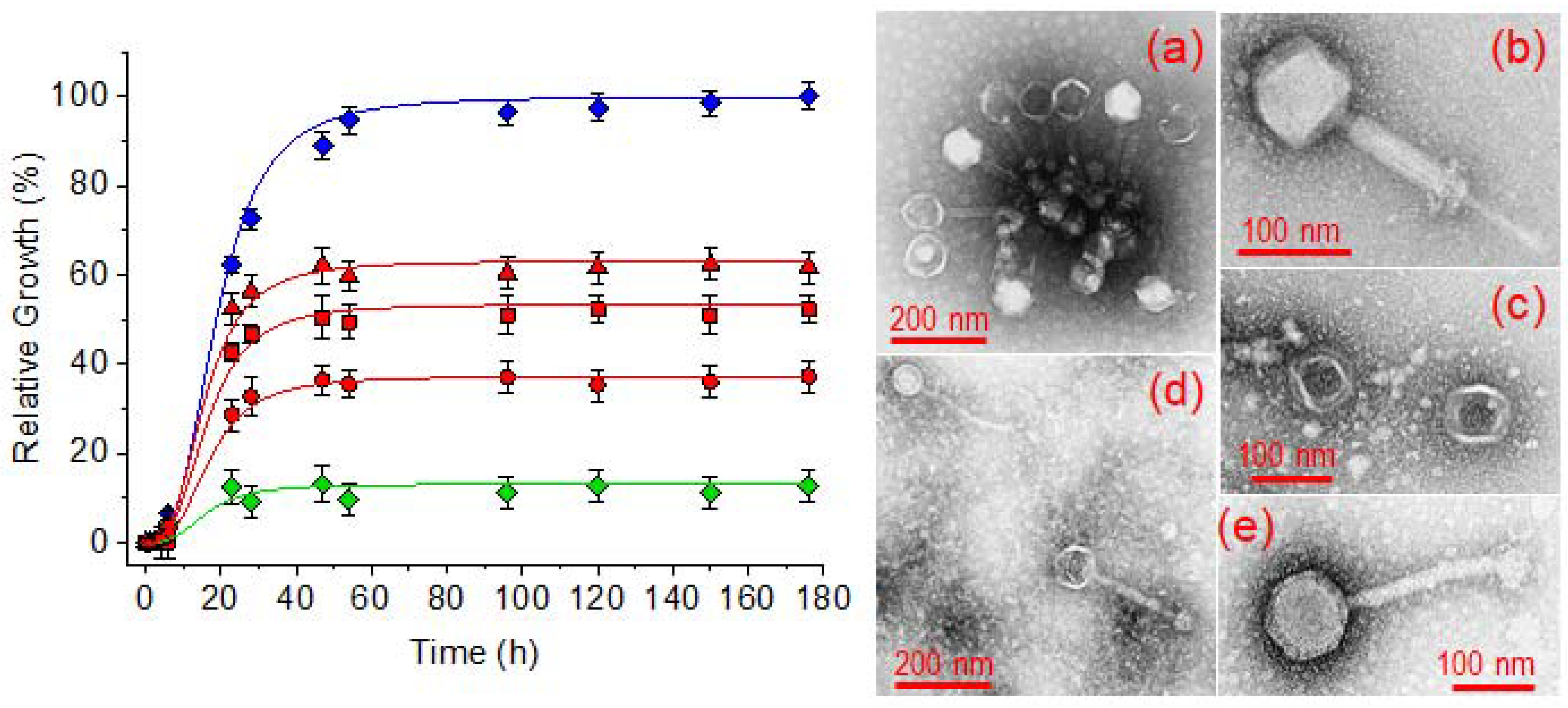
2.8. Potential of γ-PGA Hydrogels for an Efficient Load of Bacteriophages
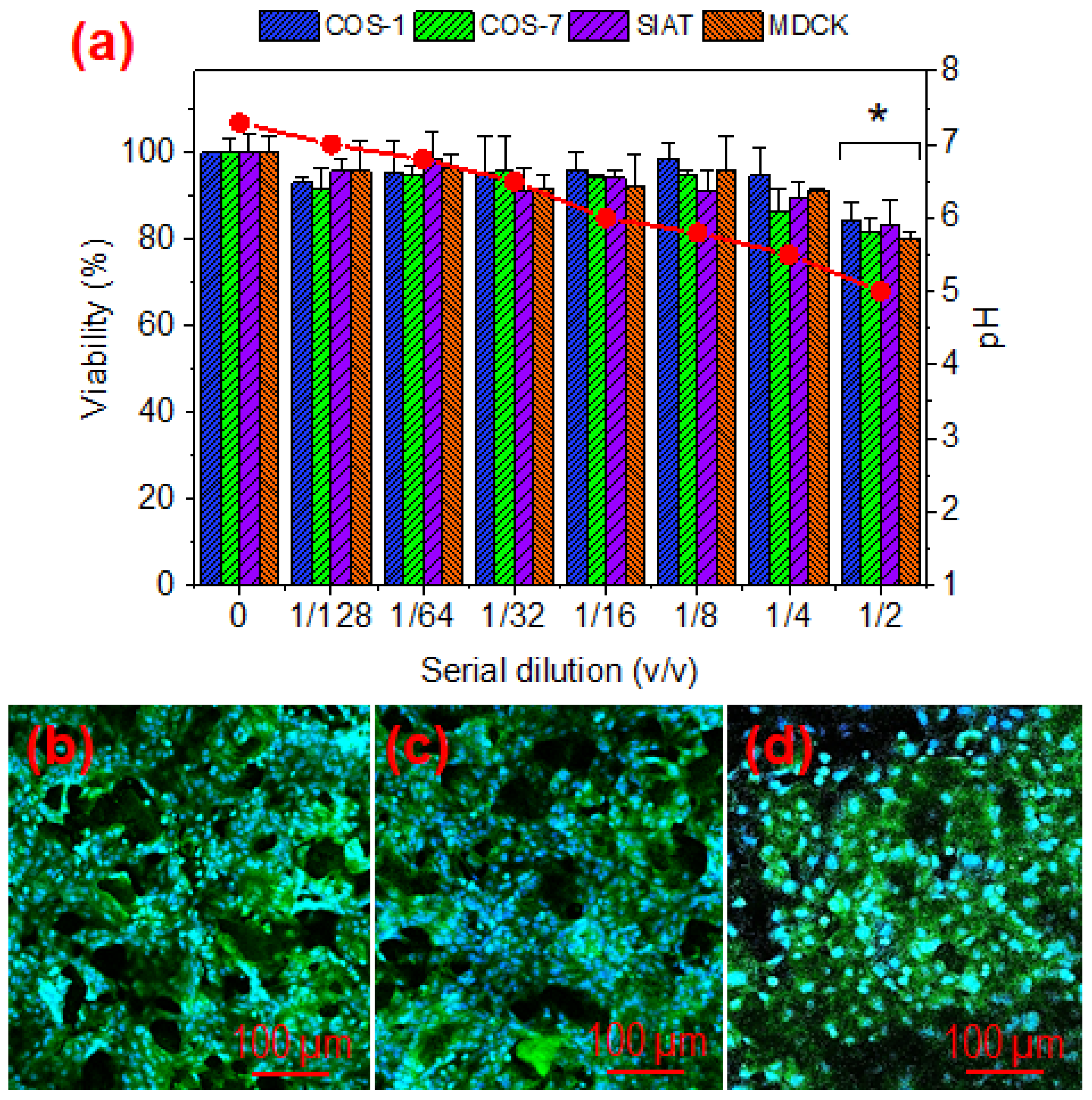
2.9. Cytotoxicity of γ-PGA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of γ-PGA Bulk Hydrogels
4.3. Electrospinning of γ-PGA Nanofibers
4.4. Preparation of Hydrogels from Electrospun γ-PGA Nanofibers
4.5. Characterization
4.6. Hydrolytic Degradation of Hydrogels
4.7. Drug Loading
4.8. Drug Release Studies from γ-PGA Hydrogels
4.9. Bactericidal Activity of the Drugs Loaded into the Electrospun γ-PGA Hydrogels
4.10. Bioactivity of Bacteriophages Loaded in Electrospun γ-PGA Hydrogels
4.11. Cytotoxicity of γ-PGA Hydrogels
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R. Biomaterials and biomedical engineering. Chem. Eng. Sci. 1995, 50, 4109–4121. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mequanint, K. Hydrogel biomaterials. In Hydrogel Biomaterials; Fazel-Rezai, R., Ed.; Biomedical Engineering-Frontiers and Challenges; InTech Open: London, UK, 2011; Chapter 14; pp. 276–295. [Google Scholar] [CrossRef] [Green Version]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 27, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Sun, G. Prevention of hospital and community acquired infections by using antibacterial textiles and clothing. In Polymeric Materials with Antimicrobial Activity; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; RSC Polymer Chemistry Series; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Jarvis, W.R. Selected aspects of the socioeconomic impact of nosocomial infections: Morbidity, mortality, cost, and prevention. Infect. Control. Hosp. Epidemiol. 1996, 17, 552–557. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.E. Economic impact of antimicrobial resistance. Emerg. Infect. Dis. 2001, 7, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Champ, S.; Huglin, M.B. Network and swelling parameters of chemically crosslinked thermoreversible hydrogels. Polymer 2001, 42, 3665–3669. [Google Scholar] [CrossRef]
- Nakamae, K.; Miyata, T.; Hoffman, A.S. Swelling behavior of hydrogels containing phosphate groups. Die Makromol. Chem. 1992, 193, 983–990. [Google Scholar] [CrossRef]
- Matsusaki, M.; Serizawa, T.; Kishida, A.; Akashi, M. Novel Functional Biodegradable Polymer III: The Construction of Poly(γ-glutamic acid)-Sulfonate Hydrogel with Fibroblast Growth Factor-2 Activity. J. Biomed. Mater. Res. Part A 2005, 73, 485–491. [Google Scholar] [CrossRef]
- Murakami, S.; Aoki, N. Bio-Based hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with various saccharides. Biomacromolecules 2006, 7, 2122–2127. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kato, K.; Shimauchi, A.; Xu, P.; Fujita, K.-I.; Tanaka, T.; Tarui, Y.; Hirasawa, E. Physicochemical properties of cross-linked poly-gamma-glutamic acid and its flocculating activity against kaolin suspension. J. Biosci. Bioeng. 2005, 99, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Genta, I.; Dorati, R.; Modena, T.; Conti, B. Poly(gamma-glutamic acid) based thermosetting hydrogels for injection: Rheology and functional parameters evaluation. React. Funct. Polym. 2019, 140, 93–102. [Google Scholar] [CrossRef]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators: Engineering and Technology; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–14. [Google Scholar]
- Jia, Z.; Lv, X.; Hou, Y.; Wang, K.; Ren, F.; Xu, D.; Wang, Q.; Fan, K.; Xie, C.; Lu, X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioact. Mater. 2021, 6, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xie, C.; Liu, J.; Zheng, Y.; Wang, J.; Lu, X. Self-adhesive hydrogels for tissue engineering. J. Mat. Chem. B. 2021, 9, 8739–8768. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Klinkhammer, K.; Matsusaki, M.; Möller, M.; Klee, D.; Akashi, M. Disulfide-crosslinked electrospun poly(γ-glutamic acid) nonwovens as reduction-responsive scaffolds. Macromol. Biosci. 2009, 9, 568–574. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Yu, Q.; Ma, T. Preparation of reactive oxygen species-responsive antibacterial hydrogels for efficient anti-infection therapy. Mat. Lett. 2020, 263, 127254. [Google Scholar] [CrossRef]
- Gamarra-Montes, A.; Missagia, B.; Morató, J.; Muñoz-Guerra, S. Antibacterial films made of ionic complexes of poly(γ-glutamic acid) and ethyl lauroyl arginate. Polymers 2018, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, H.; Tang, H.; Yu, H.; Yan, Q.; Yang, H.; Zhang, X.; Luan, S. Electrostatic assembly functionalization of poly(γ-glutamic acid) for biomedical antibacterial applications. J. Mat. Sci. Tech. 2020, 59, 14–25. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Zhu, Y.; Kyratzis, I.L. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131, 40797. [Google Scholar] [CrossRef]
- del Valle, L.; Franco, L.; Katsarava, R.; Puiggalí, J. Electrospun biodegradable polymers loaded with bactericide agents. AIMS Mol. Sci. 2016, 3, 52–87. [Google Scholar] [CrossRef] [Green Version]
- Chee, B.S.; de Lima, G.G.; Devine, D.; Nugent, M.J. Electrospun hydrogel composites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 39–70. [Google Scholar]
- Bhat, G. Polymeric nanofibers: Recent technology advancements stimulating their growth. J. Text. Sci. Eng. 2015, 5, 1–2. [Google Scholar] [CrossRef]
- Gao, C.; Ito, S.; Obata, A.; Mizuno, T.; Jones, J.R.; Kasuga, T. Fabrication and in vitro characterization of electrospun poly (γ-glutamic acid)-silica hybrid scaffolds for bone regeneration. Polymer 2016, 91, 106–117. [Google Scholar] [CrossRef]
- Garcia, J.P.D.; Hsieh, M.-F.; Doma, B.T.; Peruelo, D.C.; Chen, I.-H.; Lee, H.-M. Synthesis of gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from Camellia sinensis. Polymers 2014, 6, 39–58. [Google Scholar] [CrossRef]
- Tajima, T.; Sukigara, S. Effect of alum treatment on the mechanical and antibacterial properties of poly-g-glutamic acid nanofibers. Text. Res. J. 2012, 82, 1211–1219. [Google Scholar] [CrossRef]
- Wang, S.; Cao, X.; Shen, M.; Guo, R.; Bányai, I.; Shi, X. Fabrication and morphology control of electrospun poly(γ-glutamic acid) nanofibers for biomedical applications. Colloids Surf. B Biointerfaces 2012, 89, 254–264. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef]
- Gilbert, P.; Pemberton, D.; Wilkinson, D.E. Synergism within polyhexamethylene biguanide biocide formulations. J. Appl. Bacteriol. 1990, 69, 593–598. [Google Scholar] [CrossRef]
- Parfitt, T. Georgia: An unlikely stronghold for bacteriophage therapy. Lancet 2005, 365, 2166–2167. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Kutter, E. Bacteriophage therapy in humans. In Bacteriophages: Biology and Applications; Kutter, E., Sulakvelidze, A., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 381–436. [Google Scholar]
- Kutter, E.; de Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef]
- Lee, S.W.; Belcher, A.M. Virus-based fabrication of micro- and nanofibers using electrospinning. J. Nano Lett. 2004, 4, 387–390. [Google Scholar] [CrossRef]
- Salalha, W.; Kuhn, J.; Dror, Y.; Zussman, E. Encapsulation of bacteria and viruses in electrospun nanofibers. Nanotechnology 2006, 17, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly (ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; del Valle, L.J.; Rodrigo, N.; Casas, M.T.; Chumburidze, G.; Katsarava, R.; Puiggalí, J. Antimicrobial activity of poly(ester urea) electrospun fibers loaded with bacteriophages. Fibers 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; Fujimoto, Z. Enzymatic degradation of poly-gamma-glutamic acid. Microbiol. Monogr. 2010, 15, 95–116. [Google Scholar] [CrossRef]
- Zurita, R.; Puiggalí, J.; Rodríguez-Galán, A. Triclosan release from coated polyglycolide threads. Macromol. Biosci. 2006, 6, 58–69. [Google Scholar] [CrossRef]
- Llorens, E.; Calderón, S.; del Valle, L.J.; Puiggalí, J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 74–84. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Wagner, J.G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J. Pharm. Sci. 1969, 58, 1253–1257. [Google Scholar] [CrossRef]
- Bhargava, H.N.; Leonard, P.A. Triclosan: Applications and safety. Am. J. Infect. Control 1996, 24, 209–218. [Google Scholar] [CrossRef]
- Kim, S.A.; Moon, H.; Lee, K.; Rhee, M.S. Bactericidal effects of triclosan in soap both in vitro and in vivo. J. Antimicrob. Chemother. 2015, 70, 3345–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.J.; Horn, M.E.; Lin, Y.-C.; Patrick, J.; Parks, P.J.; Peterson, M.L. Efficacy of concurrent application of chlorhexidine gluconate and povidone iodine against six nosocomial pathogens. Am. J. Infect. Control. 2010, 38, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, M.; Yoshida, H.; Akashi, M. The construction of 3D-engineered tissues composed of cells and extracellular matrices by hydrogel template approach. Biomaterials 2007, 28, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization (Geneva, Switzerland). Available online: http://www.iso.org/standard/36406.html (accessed on 15 November 2021).


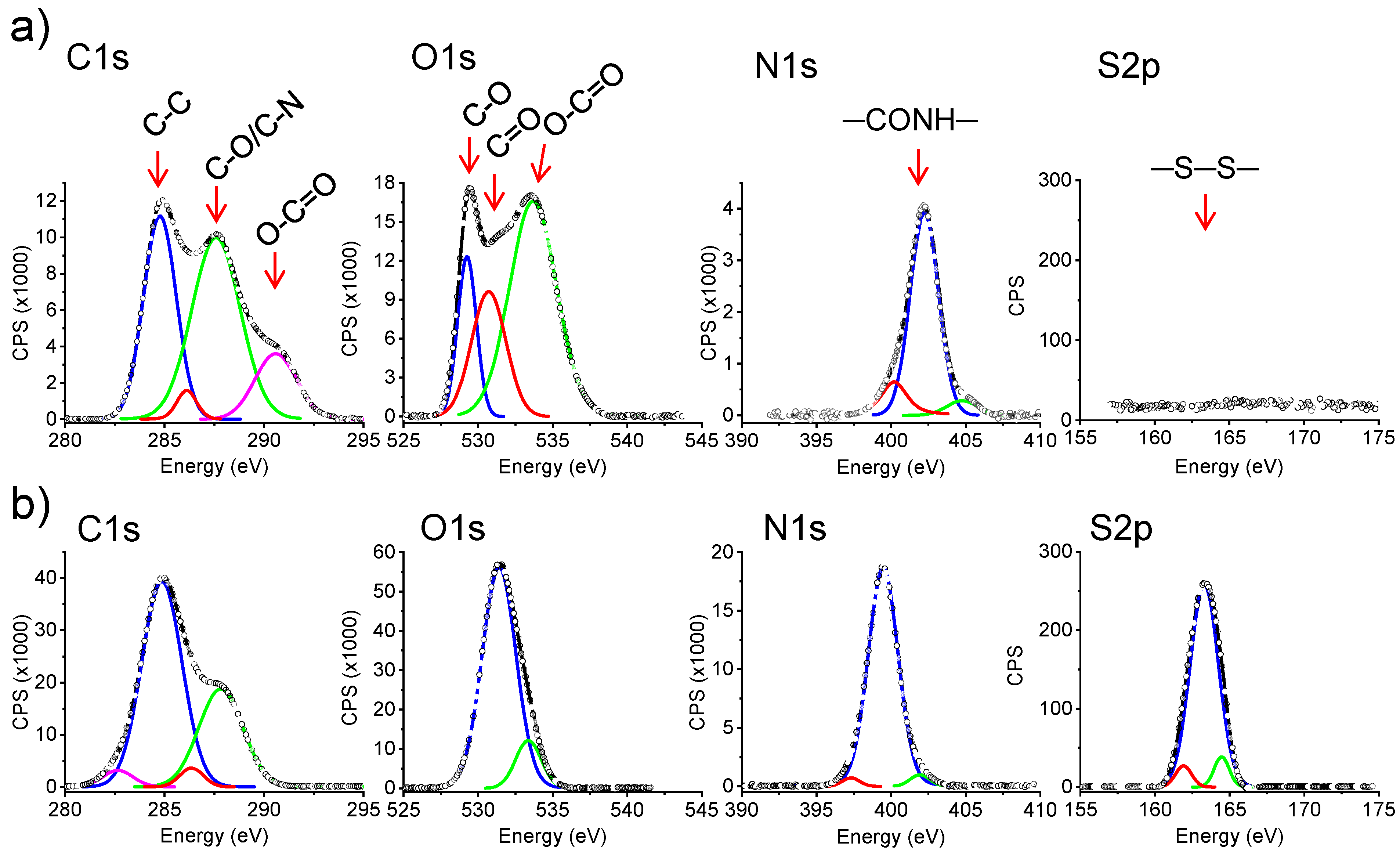


| Sample (%, Theoretical Crosslinking Degree) | C 1s (%) | O 1s (%) | N 1s (%) | S 2p (%) |
|---|---|---|---|---|
| 0% (electrospun mat) | 55.5 | 33.3 | 11.1 | 0 |
| 50% (hydrogel from electrospun fibers) | 56.2 | 29.2 | 12.5 | 2.1 |
| 75% (hydrogel from electrospun fibers) | 56.0 | 27.0 | 13.3 | 3.3 |
| 100% (hydrogel from electrospun fibers) | 56.9 | 25.5 | 13.7 | 3.9 |
| 100% (bulk hydrogel) | 57.4 | 22.2 | 14.8 | 5.0 |
| Sample (%, Theoretical Crosslinking Degree) | SU (%) |
|---|---|
| 50% (hydrogel from electrospun fibers) | 492 ± 20 |
| 75% (hydrogel from electrospun fibers) | 400 ± 17 |
| 100% (hydrogel from electrospun fibers) | 332 ± 15 |
| 100% (bulk hydrogel) | 170 ± 10 |
| Hydrogel Sample (%, Crosslinking) | EC | Drug/Loading Method | Release Medium | Higuchi Constant | First Order Constant | ||
|---|---|---|---|---|---|---|---|
| (%) | kH (h−0.5) | r | k1 (h−1) | r | |||
| 100% Bulk | 5.41 | TCS/method A | PBS/EtOH | 0.230 | 0.974 | 0.042 | 0.996 |
| 100% Electrospun | 0.32 | TCS/method A | PBS/EtOH | 1.024 | 0.980 | 1.528 | 0.980 |
| 50% Electrospun | 1.59 | TCS/method A | PBS/EtOH | 1.968 | 0.993 | 8.536 | 0.993 |
| 100% Bulk | 4.46 | TCS/method B | PBS/EtOH | 0.336 | 0.962 | 0.126 | 0.992 |
| 100% Electrospun | 0.46 | TCS/method B | PBS/EtOH | 1.590 | 0.994 | 1.443 | 0.964 |
| 50% Electrospun | 2.32 | TCS/method B | PBS/EtOH | 2.151 | 0.978 | 3.932 | 0.927 |
| 100% Electrospun | 0.07 | CHX/method A | PBS | 0.157 | 0.995 | 0.064 | 0.997 |
| 75% Electrospun | 0.12 | CHX/method A | PBS | 0.202 | 0.956 | 0.080 | 0.990 |
| 50% Electrospun | 0.19 | CHX/method A | PBS | 0.323 | 0.995 | 0.238 | 0.994 |
| 100% Electrospun | 0.78 | PHMB/method A | PBS | 0.278 | 0.988 | 0.110 | 0.981 |
| 75% Electrospun | 2.92 | PHMB/method A | PBS | 0.294 | 0.984 | 0.132 | 0.956 |
| 50% Electrospun | 4.39 | PHMB/method A | PBS | 0.304 | 0.991 | 0.307 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasbiyan, H.; Yousefzade, O.; Simiand, E.; Saperas, N.; del Valle, L.J.; Puiggalí, J. Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers. Gels 2022, 8, 120. https://doi.org/10.3390/gels8020120
Kasbiyan H, Yousefzade O, Simiand E, Saperas N, del Valle LJ, Puiggalí J. Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers. Gels. 2022; 8(2):120. https://doi.org/10.3390/gels8020120
Chicago/Turabian StyleKasbiyan, Hamidreza, Omid Yousefzade, Estelle Simiand, Núria Saperas, Luis J. del Valle, and Jordi Puiggalí. 2022. "Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers" Gels 8, no. 2: 120. https://doi.org/10.3390/gels8020120
APA StyleKasbiyan, H., Yousefzade, O., Simiand, E., Saperas, N., del Valle, L. J., & Puiggalí, J. (2022). Antibacterial Hydrogels Derived from Poly(γ-glutamic acid) Nanofibers. Gels, 8(2), 120. https://doi.org/10.3390/gels8020120








