An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Volatile Sampling
2.2.1. Solid Phase Micro Microextraction (SPME)
2.2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.2.3. Statistical and Multivariate Analyses
2.3. Transcriptomic
2.3.1. RNA Extraction for Transcriptomic Analysis
2.3.2. cDNA Library Construction and Illumina Sequencing
2.3.3. De Novo Transcriptome Assembly
2.3.4. Gene Annotation and Classification
3. Results
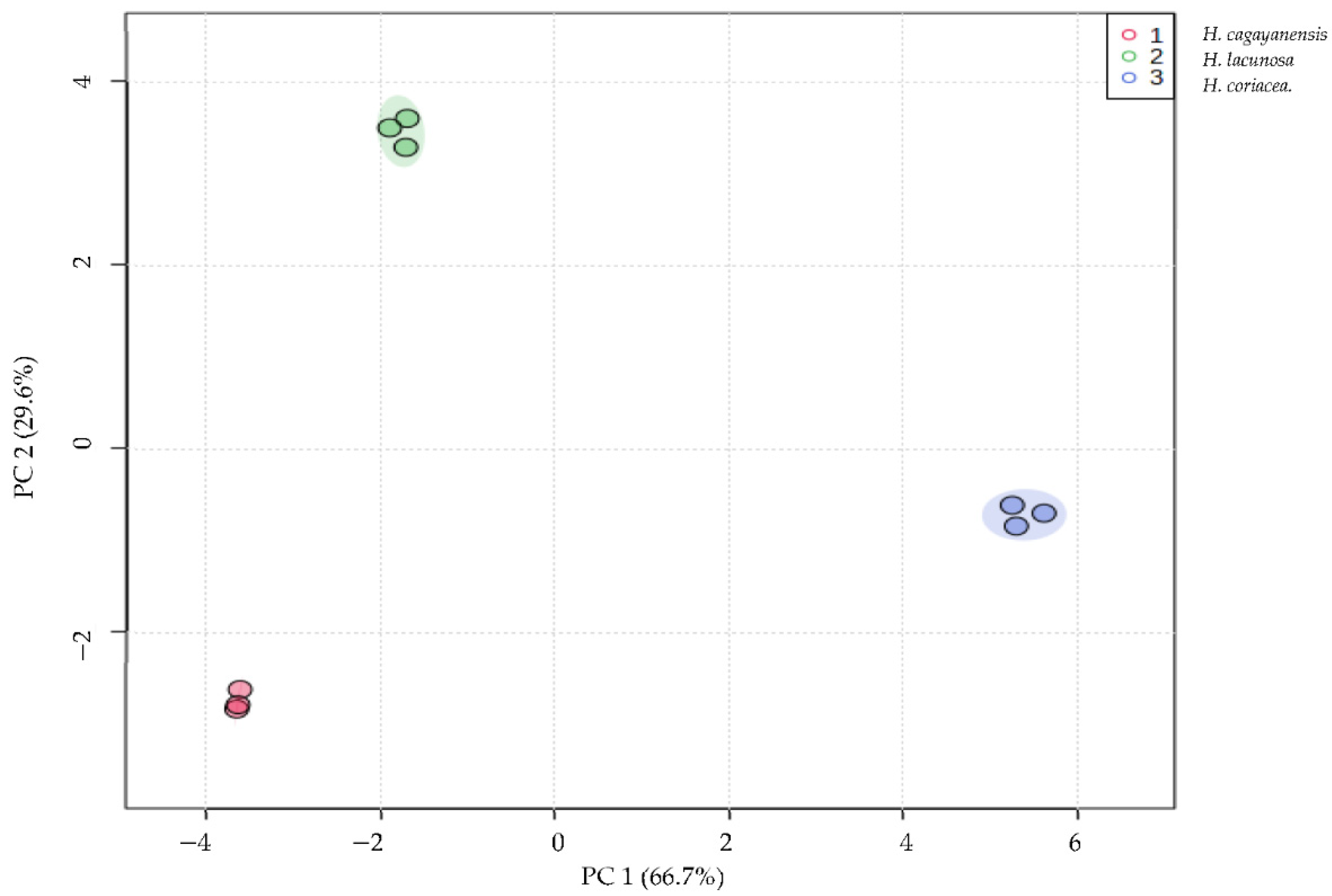
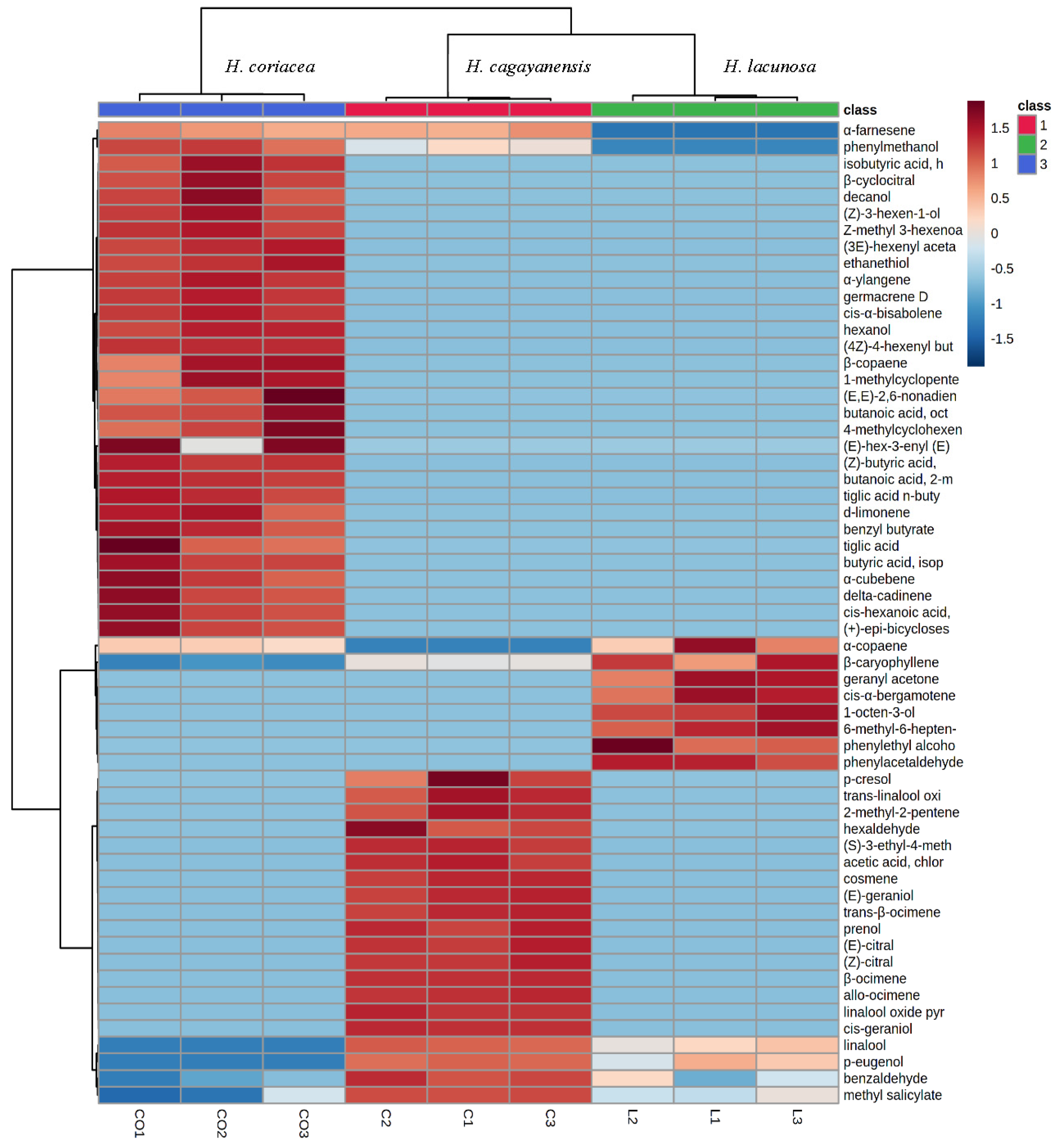
3.1. Phytochemical Analysis
3.2. Transcriptome Analysis
3.2.1. Assembly of Flower Hoya Species Transcriptomes
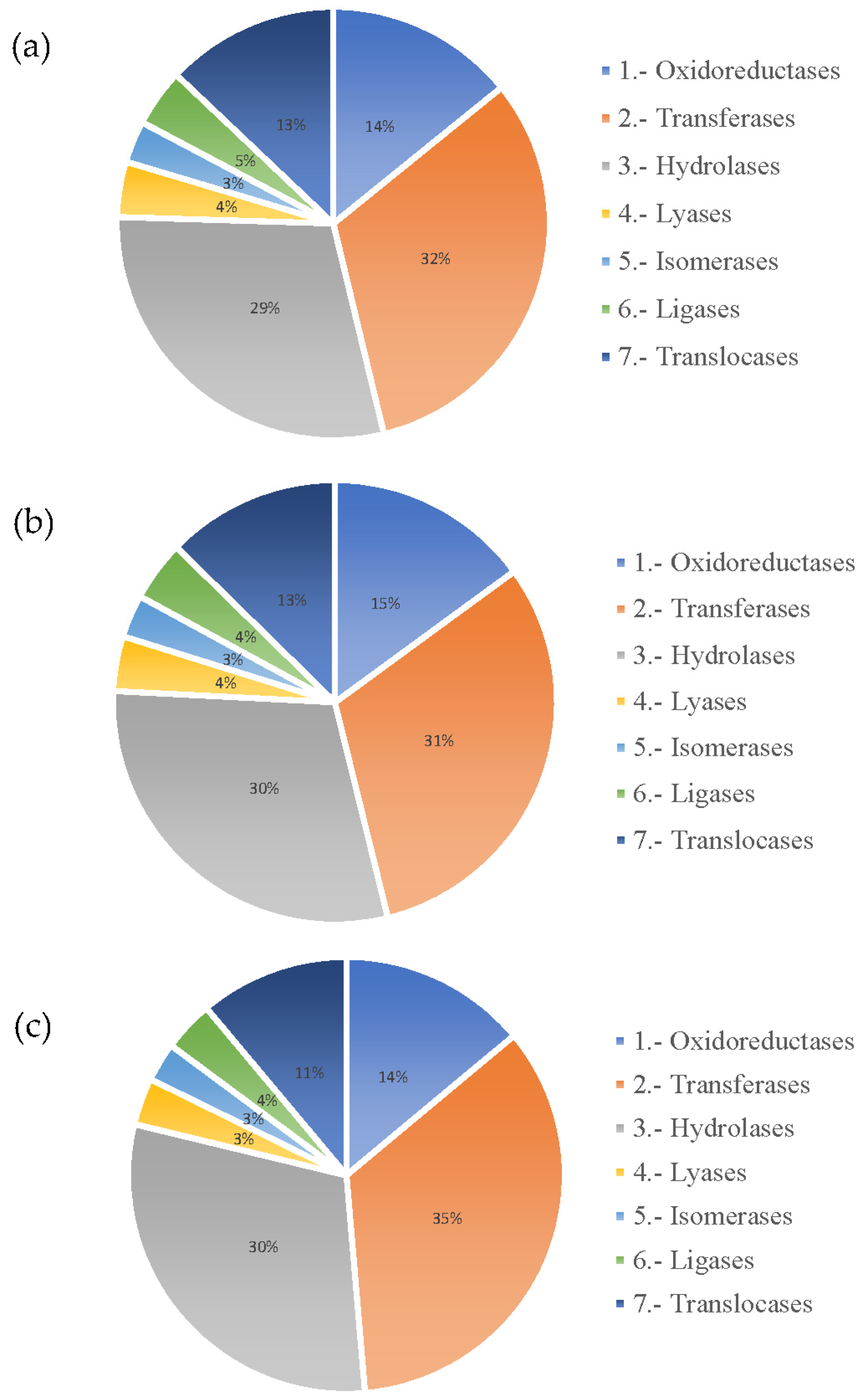
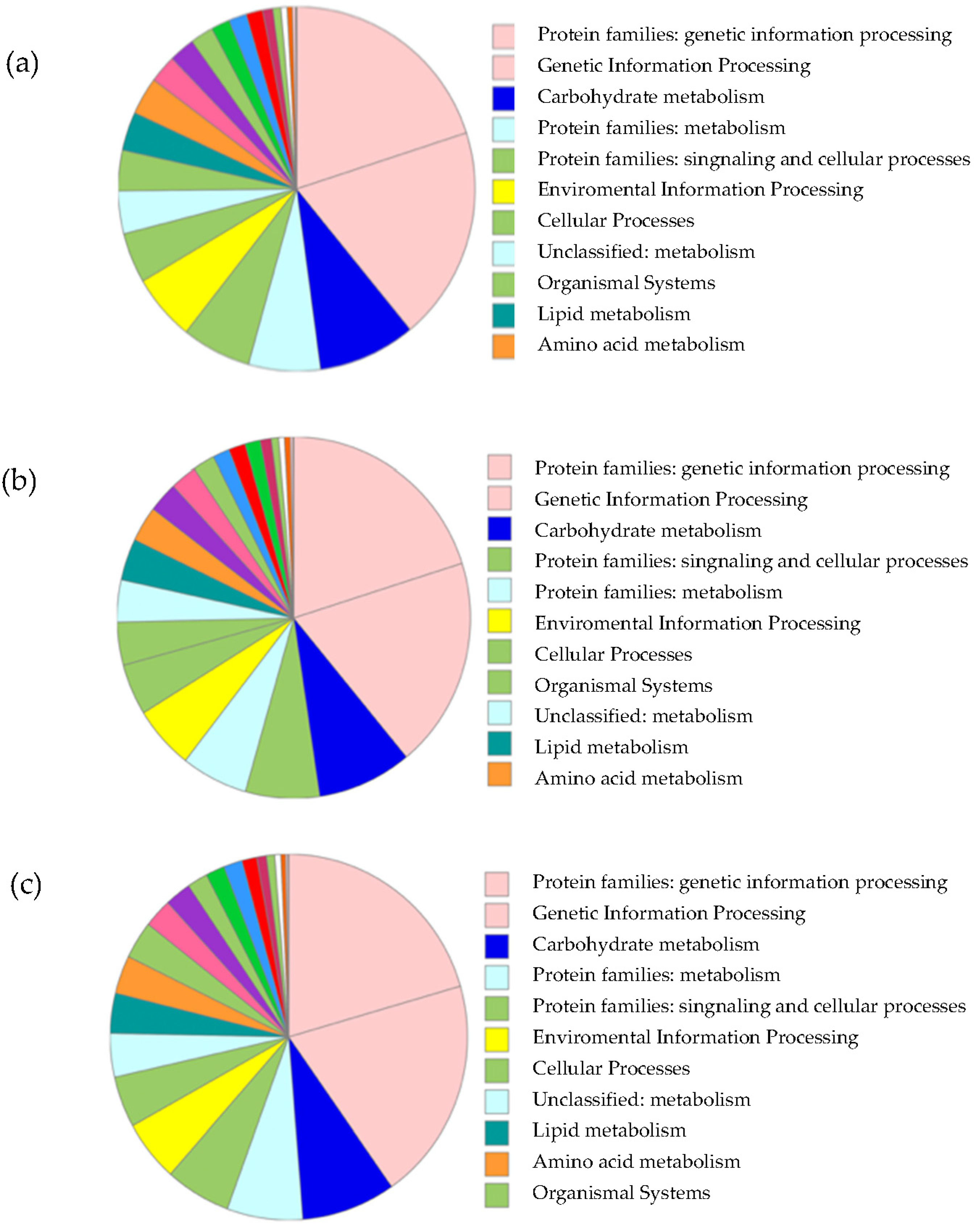
3.2.2. Annotation of Unigenes
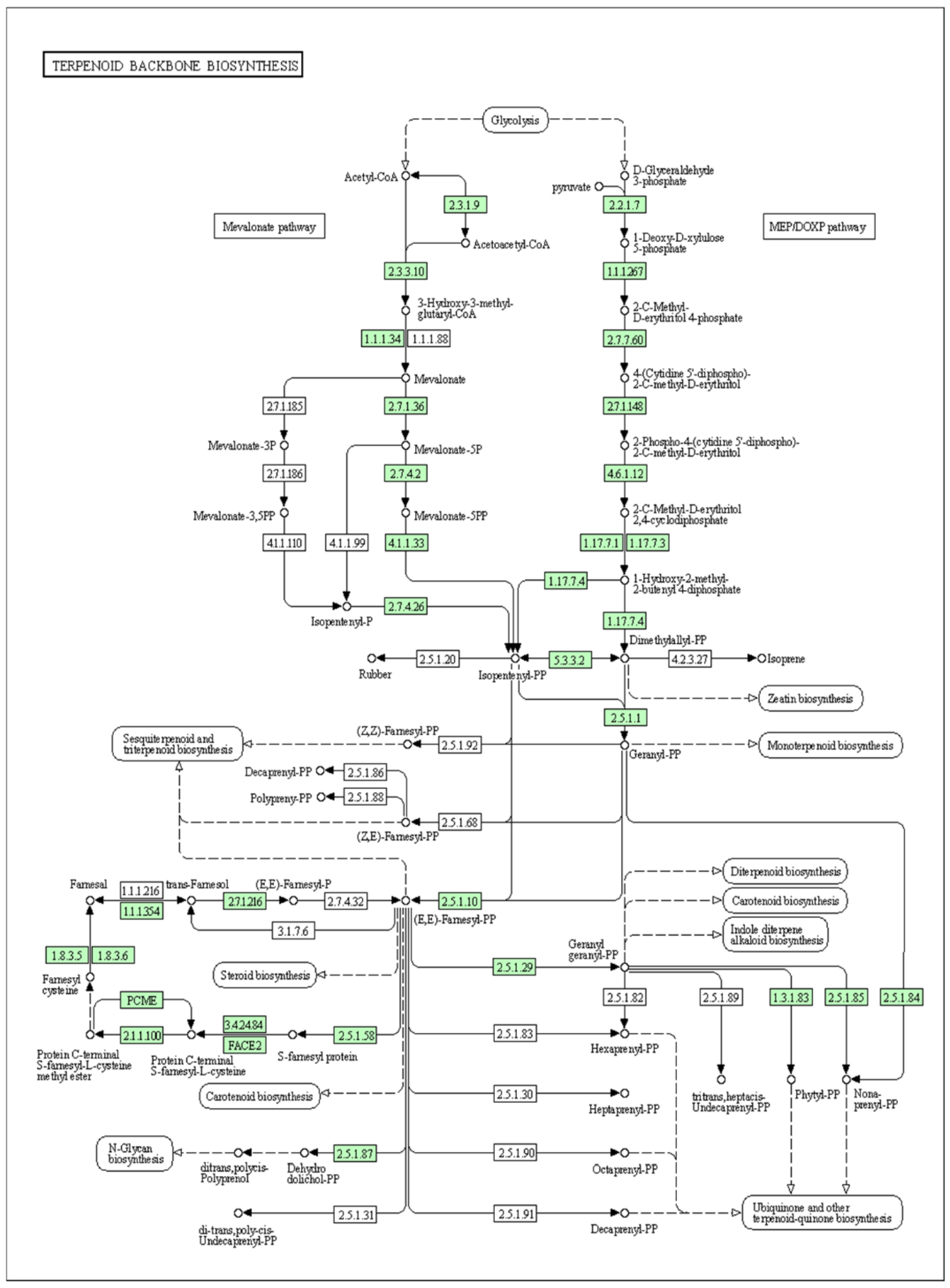
3.2.3. Transcriptome Analysis of Secondary Metabolite Biosynthesis Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebajo, V.D., Jr.; Shen, C.C.; Ragasa, C.Y. Terpenoids and sterols from Hoya multiflora Blume. J. Appl. Pharm. Sci. 2015, 5, 33–39. [Google Scholar]
- Brown, R. On the Asclepiadeae, a natural order of plants separated from the Apocineae of Jussieu. Mem. Wernerian Nat. Hist. Soc. 1810, 1, 26–27. [Google Scholar]
- Kleijn, D.; van Donkelaar, R. Notes on the taxonomy and ecology of the genus Hoya (Asclepiadaceae) in Central Sulawesi. Blumea Biodivers. Evol. Biogeogr. Plants 2001, 46, 457–483. [Google Scholar]
- Wanntorp, L.; Kunze, H. Identifying synapomorphies in the flowers of Hoya and Dischidia, Toward phylogenetic understanding. Int. J. Plant Sci. 2009, 170, 331–342. [Google Scholar] [CrossRef]
- Liddle, D.J. New species Hoya lockii (Apocynaceae, Asclepiadoideae). Taiwania 2009, 57, 49–54. [Google Scholar]
- Averyanov, L.V.; Averyanova, A.L.; Nguyen, K.S.; Maisak, T.V. Hoya aphylla (Apocynaceae, Asclepiadoideae), a new leafless species from Laos. Nord. J. Bot. 2019, 37, 56–62. [Google Scholar] [CrossRef]
- He, S.Y.; Wu, W.; Li, P.T.; Zeng, M.L. Hoya daimenglongensis (Apocynaceae, Asclepiadoideae), a new species from Yunnan, China. J. Bot. Nomencl. 2012, 22, 170–173. [Google Scholar] [CrossRef]
- Kloppenburg, R.D.; Guevarra, M.L.D.; Carandang, J.M.; Maranan, F.S. New Species of Hoya R. Br. (Apocynaceae) from the Philippines. J. Nat. Stud. 2012, 11, 34–48. [Google Scholar]
- Pham, V.T.; Le, T.A.; Averyanov, L. Hoya hanhiae sp. nov.(Apocynaceae, Asclepiadoideae) from central Vietnam. Nord. J. Bot. 2015, 33, 64–67. [Google Scholar] [CrossRef]
- Rahayu, S.; Rodda, M. Hoya of Sumatra, an updated checklist, three new species, and a new subspecies. Eur. J. Taxon. 2019, 508, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Rodda, M.; Rahmad, Z. Hoya peninsularis (Apocynaceae, Asclepiadoideae), a new species from Peninsular Malaysia, and notes on Hoya maingayi and Gongronema wrayi. Nord. J. Bot. 2020, 38, 42–52. [Google Scholar] [CrossRef]
- Rodda, M.; Juhonewe, N.S. Hoya medinillifolia (Apocynaceae Asclepiadoideae), a new species from lowland forests of Sarawak, Borneo. Webbia 2011, 66, 149–154. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Panajon, N.M.; Aurigue, F.B.; Brkljača, R.; Urban, S. Chemical constituents of Hoya cumingiana Decne. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 2033–2038. [Google Scholar]
- Ragasa, C.Y.; Borlagdan, M.S.; Aurigue, F.B.; Brkljaca, R.; Urban, S. Chemical constituents of Hoya cagayanensis C.M. Burton. J. Appl. Pharm. Sci. 2017, 7, 61–65. [Google Scholar]
- Van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Rahman, A.; Goh, H.H.; Loke, K.K.; Noor, N.M.; Aizat, W.M. RNA-seq analysis of mangosteen (Garcinia mangostana L.) fruit ripening. Genom. Data 2017, 12, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; MacManes, M.D. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; Pesseat, S. InterProscan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Boil. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundan, M.; Gani, U.; Nautiyal, A.K.; Misra, P. Molecular Biology of Glandular Trichomes and their functions in environmental stresses. In Molecular Approaches in Plant Biology and Environmental Challenge; Singh, S.P., Upadhyay, S.K., Pandey, A., Kumar, S., Eds.; Springer: Singapore, 2019; pp. 365–393. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Grison-Pigé, L.; Hossaert-McKey, M.; Greeff, J.M.; Bessière, J.M. Fig volatile compounds a first comparative study. Phytochemistry 2002, 61, 61–71. [Google Scholar] [CrossRef]
- Negre-Zakharov, F.; Long, M.C.; Dudareva, N. Floral Scents and Fruit Aromas Inspired by Nature. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 405–430. [Google Scholar]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Donaldson, J.M.I.; Mcgovern, T.P.; Ladd, T.L. Floral attractants for Cetoniinae and Rutelinae (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1990, 83, 1298–1305. [Google Scholar] [CrossRef]
- Larsson, M.C.; Stensmyr, M.C.; Bice, S.B.; Hansson, B.S. Attractiveness of fruit and flower odorants detected by olfactory receptor neurons in the fruit chafer Pachnoda Marginata. J. Chem. Ecol. 2003, 29, 1253–1268. [Google Scholar] [CrossRef]
- Granero, A.M.; Guerra Sanz, J.M.; Egea Gonzalez, F.J.; Martinez Vidal, J.L.; Dornhaus, A.; Ghani, J.; Serrano, A.R.; Chittka, L. Chemical compounds of the foraging recruitment pheromone in bumblebees. Sci. Nat. 2005, 92, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecetti, L.; Tava, A.; Felicioli, A.; Pinzauti, M.; Piano, E. Effect of three volatile compounds from lucerne flowers on their attractiveness towards pollinators. Bull. Insectoogy 2002, 55, 21–27. [Google Scholar]
- Hills, H.G.; Williams, N.H.; Dodson, C.H. Floral fragrances and isolating mechanisms in the genus Catasetum (Orchidaceae). Bio Trop. 1972, 4, 61–76. [Google Scholar] [CrossRef]
- Bendera, M.; Ekesi, S.; Ndung’u, M.; Srinivasan, R.; Torto, B. A major host plant volatile, 1-octen-3-ol, contributes to mating in the legume pod borer, Maruca vitrata (Fabricius)(Lepidoptera: Crambidae). Sci. Nat. 2015, 102, 47. [Google Scholar] [CrossRef]
- Fu, J.; Hou, D.; Wang, Y.; Zhang, C.; Bao, Z.; Zhao, H.; Hu, S. Identification of floral aromatic volatile compounds in 29 cultivars from four groups of Osmanthus fragrans by gas chromatography–mass spectrometry. Hortic. Environ. Biotechnol. 2019, 60, 611–623. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biology of Floral Scent; CRC Press: London, UK, 2006. [Google Scholar]
- Jürgens, A.; Dötterl, S.; Liede-Schumann, S.; Meve, U. Floral scent composition in early diverging taxa of Asclepiadoideae, and Secamonoideae (Apocynaceae). S. Afr. J. Bot. 2010, 76, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Pacini, E.; Nicolson, W.S. Introduction. In Nectaries and Nectar; Nicolson, W.S., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–111. [Google Scholar]
- Beath, D.D.N. Pollination of Armorphophallus johnsonii (Araceae) by carrion bettles (Phaeochrous amplus) in a Ghanaian rain forest. J. Trop. Ecol. 1996, 3, 409–418. [Google Scholar] [CrossRef]
- Van der Niet, T.; Hansen, D.M.; Johnson, S.D. Carrion mimicry in a South African orchid: Flowers attract a narrow subset of the fly assembly on animal carcasses. Ann. Bot. 2011, 107, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef]
- Bonn, B.; Moortgat, G.K. Sesquiterpene ozonolysis: Origin of atmospheric new particle formation from biogenic hydrocarbons. Geophys. Res. Lett. 2003, 30, 1585. [Google Scholar] [CrossRef]
- Byers, K.J.; Bradshaw, H.D.; Riffell, J.A. Three floral volatiles contribute to differential pollinator attraction in monkey flowers (Mimulus). J. Exp. Biol. 2014, 217, 614–623. [Google Scholar]
- Hambäck, P.A. Getting the smell of it odour cues structure pollinator networks. J. Anim. Ecol. 2016, 85, 315–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas-Barbosa, D.; Sun, P.; Hakman, A.; van Beek, T.A.; van Loon, J.J.A.; Dicke, M. Visual and odour cues: Plant responses to pollination and herbivory affect the behaviour of flower visitors. Funct. Ecol. 2016, 30, 431–441. [Google Scholar] [CrossRef]
- Bernotienë, G.; Nivinskienë, O.; Butkienë, R.; Mockutë, D. Chemical composition of essential oils of hops (Humulus lupulus L.) growing wild in Aukstaitija. Chemija 2004, 15, 31–36. [Google Scholar]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Zviely, M.; Li, M. Ocimene a versatile floral ingredient. In Flavor Ingredients and Formulation; Allured, J.G., Ed.; hlm. 42–45; Allured Business Media: Carol Stream, IL, USA, 2013. [Google Scholar]
- Knudsen, J.T.; Tollsten, L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth-pollinated taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; Gershenzon, J.; Unsicker, S.B. Floral odor bouquet loses its ant repellent properties after inhibition of terpene biosynthesis. J. Chem. Ecol. 2011, 37, 1323–1331. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2001, 193, 997–1008. [Google Scholar] [CrossRef]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Sarmin, N.I.M.; Ng, C.L.; Tiong, J.J.; Aizat, W.M.; Keong, L.K.; Zin, N.M. Genomic characterization of a new endophytic Streptomyces kebangsaanensis identifies biosynthetic pathway gene clusters for novel phenazine antibiotic production. PeerJ 2017, 5, 3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.W.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Tsai, W.C.; Kuoh, C.S.; Huang, T.H.; Wang, H.C.; Wu, T.S.; Chen, H.H. Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biol. 2006, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.I.; Mook-Jung, I. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 2019, 30, 493–507. [Google Scholar] [CrossRef]
- Mohd-Hairul, A.R.; Namasivayam, P.; Lian, G.E.C.; Abdullah, J.O. Terpenoid, benzenoid, and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Ramya, M.; Jang, S.; An, H.R.; Lee, S.Y.; Park, P.M.; Park, P.H. Volatile organic compounds from orchids: From synthesis and function to gene regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef] [Green Version]
- Dürr, C.; Schnell, H.J.; Luzhetskyy, A.; Murillo, R.; Weber, M.; Welzel, K.; Bechthold, A. Biosynthesis of the terpene phenalinolactone in Streptomyces sp. Tü6071: Analysis of the gene cluster and generation of derivatives. Chem. Biol. 2006, 13, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuizen, N.J.; Wang, M.Y.; Matich, A.J.; Green, S.A.; Chen, X.; Yauk, Y.K.; Atkinson, R.G. Two terpene synthases are responsible for the major sesquiterpenes emitted from the flowers of kiwifruit (Actinidia deliciosa). J. Exp. Bot. 2009, 60, 3203–3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]



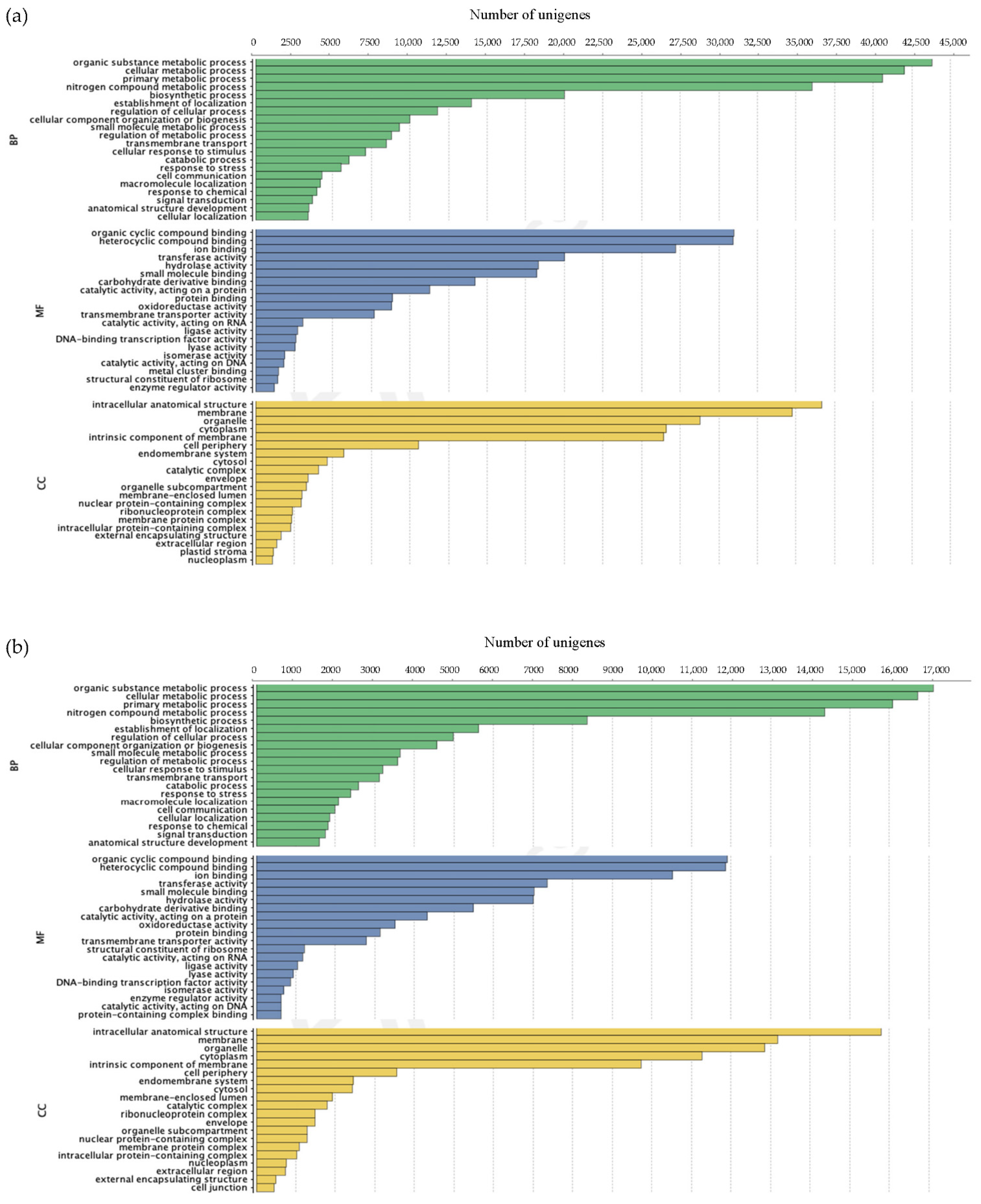
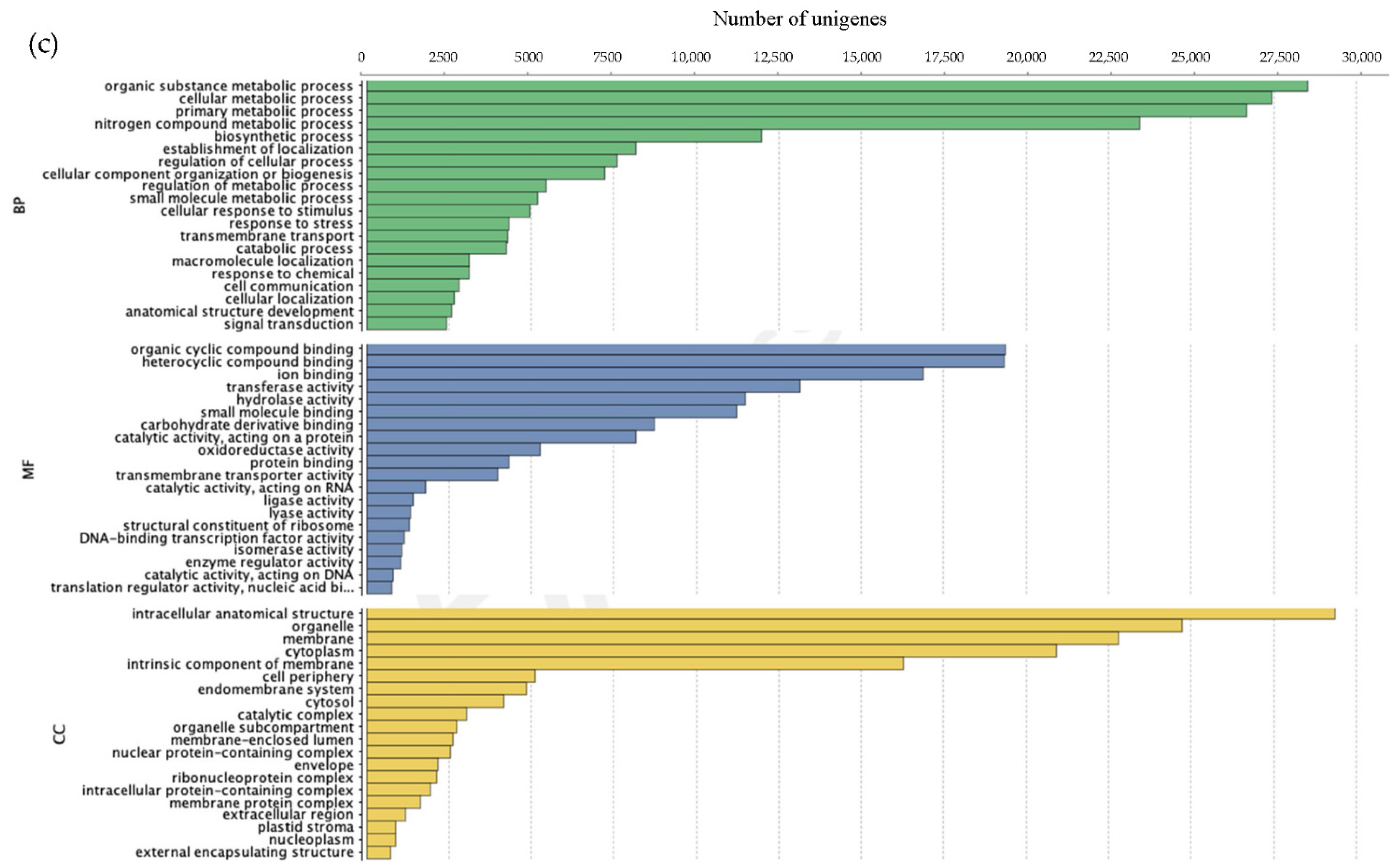
| Species | Sample ID | Location Site, State | Coordinates |
|---|---|---|---|
| H. cagayanensis | HA 01 | Baling, Kedah | 5°30′06.8″ N 100°46′19.1″ E |
| H. lacunosa | HA 02 | Baling, Kedah | 5°34′02.7″ N 100°52′05.8″ E |
| H. coriacea | HA 03 | Sik, Kedah | 5°51′42.2″ N 100°50′11.1″ E |
| Formula and Chemical Group | CAS. No | Compound | Plant Species/ Relative Percentage (%) | ||
|---|---|---|---|---|---|
| H. cagayanensis | H. lacunosa | H. coriacea | |||
| Terpenoids | |||||
| Monoterpene | |||||
| C10H16 | 013877-91-3 | β-Ocimene | 25.78 | - | - |
| C10H16 | 005989-27-5 | d-Limonene | - | - | 0.05 |
| C10H18O | 000106-24-1 | (E)-Geraniol | 1.64 | - | - |
| C10H18O | 000106-25-2 | cis-Geraniol | 0.28 | - | - |
| C10H18O | 000078-70-6 | Linalool | 9.34 | 3.22 | - |
| C10H16O | 000141-27-5 | (E)-Citral | 0.33 | - | - |
| C10H16O | 000106-26-3 | (Z)-Citral | 0.27 | - | - |
| C10H16O | 000432-25-7 | β-Cyclocitral | - | - | 0.04 |
| C10H16 | 003779-61-1 | trans-β-Ocimene | 0.54 | - | - |
| C10H18O2 | 034995-77-2 | trans-Linalool oxide (furanoid) | 0.14 | - | - |
| C10H16 | 007216-56-0 | Allo-ocimene | 0.79 | - | - |
| Sesquiterpene | |||||
| C15H24 | 017699-14-8 | α-Cubebene | - | - | 0.18 |
| C15H24 | 1000360-33-0 | α-Copaene | - | 0.44 | 0.17 |
| C15H24 | 1000374-19-0 | α-Ylangene | - | - | 0.02 |
| C15H24 | 1000374-18-9 | β-Copaene | - | - | 0.07 |
| C15H24 | 000502-61-4 | α-Farnesene | 0.18 | - | 0.19 |
| C15H24 | 000087-44-5 | β-Caryophyllene | 0.22 | 1.00 | 0.06 |
| C15H24 | 017699-05-7 | cis-α-Bergamotene | - | 0.37 | - |
| C15H24 | 023986-74-5 | Germacrena D | - | - | 1.01 |
| C15H24 | 029837-07-8 | cis-α-Bisabolene | - | - | 0.03 |
| C15H24 | 000483-75-0 | delta-Cadinene | - | - | 0.65 |
| Alcohols | |||||
| C6H12O | 000928-96-1 | (Z)-3-Hexen-1-ol | - | 0.55 | 19.92 |
| C6H14O | 000111-27-3 | Hexanol | - | - | 1.16 |
| C8H16O | 003391-86-4 | 1-Octen-3-ol | - | 26.1 | - |
| C10H18O2 | 014049-11-7 | Linalool oxide pyranoside | 2.03 | 0.74 | 0.07 |
| C10H18O | 004117-14-0 | Decanol | - | - | 0.22 |
| C8H10O | 000060-12-8 | Phenylethyl alcohol | - | 2.20 | - |
| C5H10O | 000556-82-1 | Prenol | 0.7 | - | - |
| C8H18O | 1000144-07-1 | (S)-3-Ethyl-4-methylpentanol | 7.66 | - | - |
| Ester | |||||
| C8H14O2 | 003681-82-1 | (3E)-Hexenyl acetate | - | - | 0.16 |
| C11H18O2 | 1000373-74-1 | (E)-Hex-3-enyl (E)-2-methylbut-2-enoate | - | - | 8.14 |
| C10H18O2 | 069727-41-9 | (4Z)-4-Hexenyl butyrate | - | - | 0.61 |
| C7H13ClO2 | 005326-92-1 | Acetic acid, chloro-,3-methylbutyl ester | 1.22 | - | - |
| C11H20O2 | 053398-85-9 | Butanoic acid (Z)-2-methyl-3-hexenyl ester, | - | - | 11.78 |
| C10H18O2 | 016491-36-4 | (Z)-Butyric acid, 3-hexenyl ester | - | - | 29.36 |
| C12H24O2 | 000110-39-4 | Butanoic acid, octyl ester | - | - | 3.15 |
| C12H22O2 | 031501-11-8 | cis-Hexanoic acid, 3-hexenyl ester | - | - | 1.43 |
| C9H18O2 | 000106-27-4 | Butyric acid, isopentyl ester | - | - | 0.31 |
| C7H12O2 | 013894-62-7 | Z-Methyl 3-hexenoate | - | - | 0.06 |
| C9H16O2 | 007785-66-2 | Tiglic acid n-butyl ester | - | - | 0.05 |
| C10H20O2 | 002349-07-7 | Isobutyric acid, hexyl ester | - | - | 0.4 |
| Benzenoid | |||||
| C6H5CHO | 000100-52-7 | Benzaldehyde | 5.7 | 2.11 | 1.3 |
| C11H14O2 | 000103-37-7 | Benzyl butyrate | - | - | 0.31 |
| C10H12O2 | 000097-53-0 | p-Eugenol | 7.1 | 2.55 | - |
| C8H8O3 | 000119-36-8 | Methyl salicylate | 24.67 | 1.75 | 0.66 |
| C7H8O | 000100-51-6 | Phenylmethanol | 0.36 | - | 2.05 |
| C7H8O | 000106-44-5 | p-Cresol | 0.13 | - | - |
| Alkenes | - | - | - | ||
| C15H24 | 054274-73-6 | (+)-epi-Bicyclosesquiphellandrene | - | - | 0.17 |
| C6H10 | 000693-89-0 | 1-Methylcyclopentene | - | 0.3 | |
| C10H14 | 000460-01-5 | Cosmene | 0.65 | - | |
| C7H12 | 000591-47-9 | 4-Methylcyclohexene | - | - | 0.03 |
| C6H12 | 000625-27-4 | 2-Methyl-2-pentene | 0.48 | - | - |
| Aldehydes | |||||
| C6H12O | 000066-25-1 | Hexaldehyde | 1.19 | - | - |
| C8H8O | 000122-78-1 | Phenylacetaldehyde | - | 2.36 | - |
| C9H14O | 017587-33-6 | (E,E)-2,6-Nonadienal | - | - | 1.73 |
| Ketone | |||||
| C8H14O | 010408-15-8 | 6-Methyl-6-hepten-2-one | - | 4.71 | - |
| C13H22O | 003796-70-1 | Geranyl acetone | - | 0.42 | - |
| Acid | |||||
| C5H8O2 | 000080-59-1 | Tiglic acid | - | - | 0.94 |
| Sulphur | |||||
| C2H6S | 000075-08-1 | Ethanethiol | - | - | 0.11 |
| Statistic | H. cagayanensis | H. lacunosa | H. coriacea |
|---|---|---|---|
| Unigenes | |||
| Total unigenes | 144,042 | 156,830 | 102,914 |
| GC% | 42.68 | 42.66 | 41.49 |
| Contig N50 (bp) | 989 | 1178 | 1545 |
| Minimum contig length (bp) | 186 | 187 | 199 |
| Maximum contig length (bp) | 9665 | 11,303 | 9825 |
| Median contig length (bp) | 350 | 315 | 547 |
| Average contig length (bp) | 619.35 | 649.54 | 909.36 |
| Total bases | 89,212,671 | 101,866,954 | 93,585,886 |
| Statistic | H. cagayanensis | H. lacunosa | H. coriacea |
|---|---|---|---|
| Unigenes Annotation | |||
| NCBI-Nr | 109,240 (75.84%) | 42,479 (69.00%) | 72,610 (70.55%) |
| KEGG | 25,186 (17.49%) | 24,703 (40.10%) | 23,752 (23.08%) |
| InterPRO | 54,331 (37.72%) | 55,079 (89.41%) | 52,269 (50.79%) |
| Gene Ontology (GO) | 89,203 (61.93%) | 33,581 (54.52%) | 56,914 (55.30%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basir, S.; Akbar, M.A.; Talip, N.; Baharum, S.N.; Bunawan, H. An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae 2022, 8, 224. https://doi.org/10.3390/horticulturae8030224
Basir S, Akbar MA, Talip N, Baharum SN, Bunawan H. An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae. 2022; 8(3):224. https://doi.org/10.3390/horticulturae8030224
Chicago/Turabian StyleBasir, Syazwani, Muhamad Afiq Akbar, Noraini Talip, Syarul Nataqain Baharum, and Hamidun Bunawan. 2022. "An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae)" Horticulturae 8, no. 3: 224. https://doi.org/10.3390/horticulturae8030224
APA StyleBasir, S., Akbar, M. A., Talip, N., Baharum, S. N., & Bunawan, H. (2022). An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae, 8(3), 224. https://doi.org/10.3390/horticulturae8030224






