One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis
Abstract
1. Introduction
2. The Origins of Hematopoiesis: Hemangioblast and Hemogenic Endothelium
2.1. Before the Hemogenic Endothelium: The Hemangioblast Theory
2.2. Heterogeneity of the Hemogenic Endothelium
3. Heterogeneity of Hematopoietic Stem Cell (HSC)-Independent Hematopoiesis
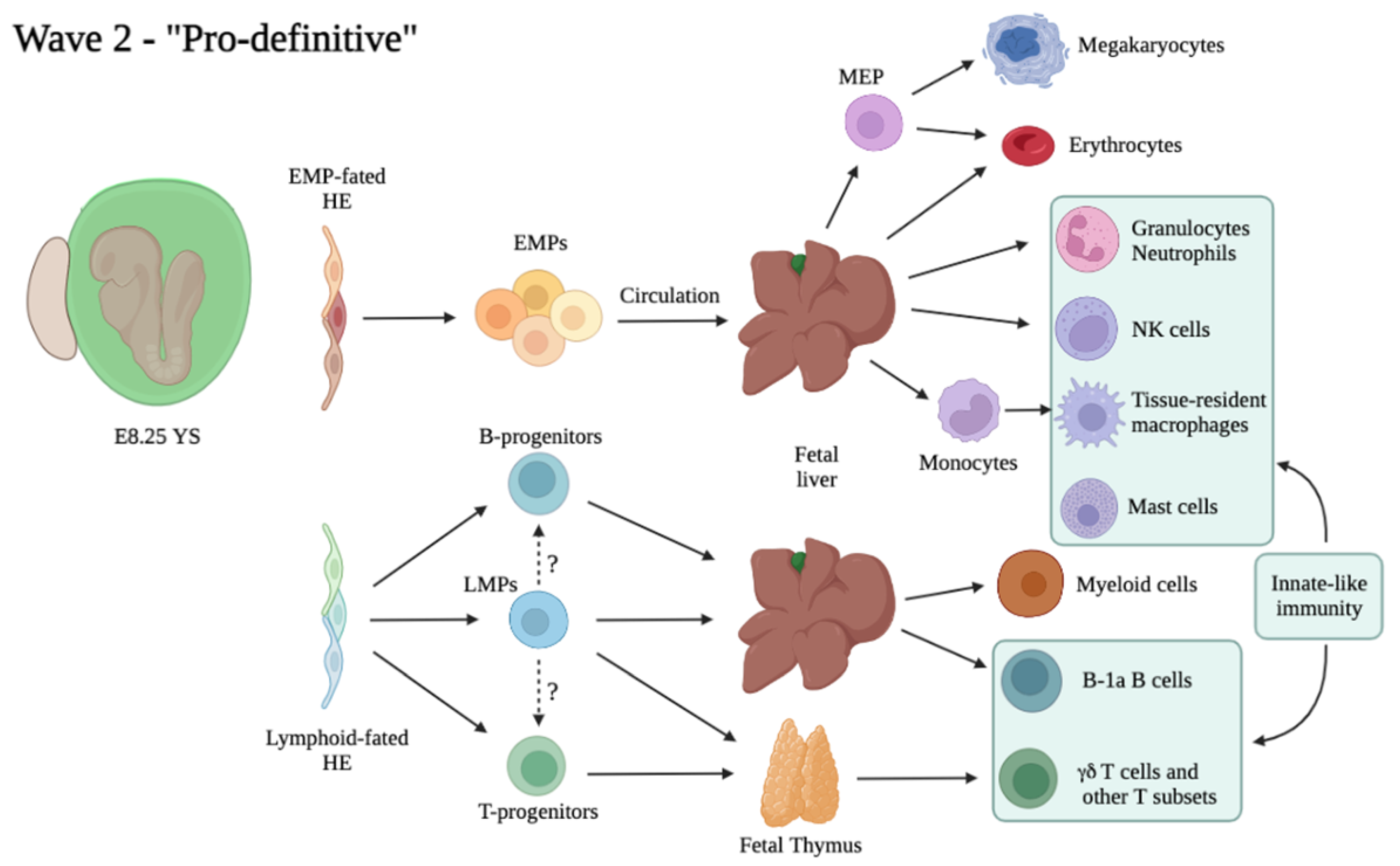
3.1. Multiple Waves of HSC-Independent Progenitors
3.2. “Primitive” Hematopoiesis: One Wave, Different Origins?
3.3. The “Second Wave”: Appearance of Multi-Potent Hematopoietic Progenitors in the Embryo
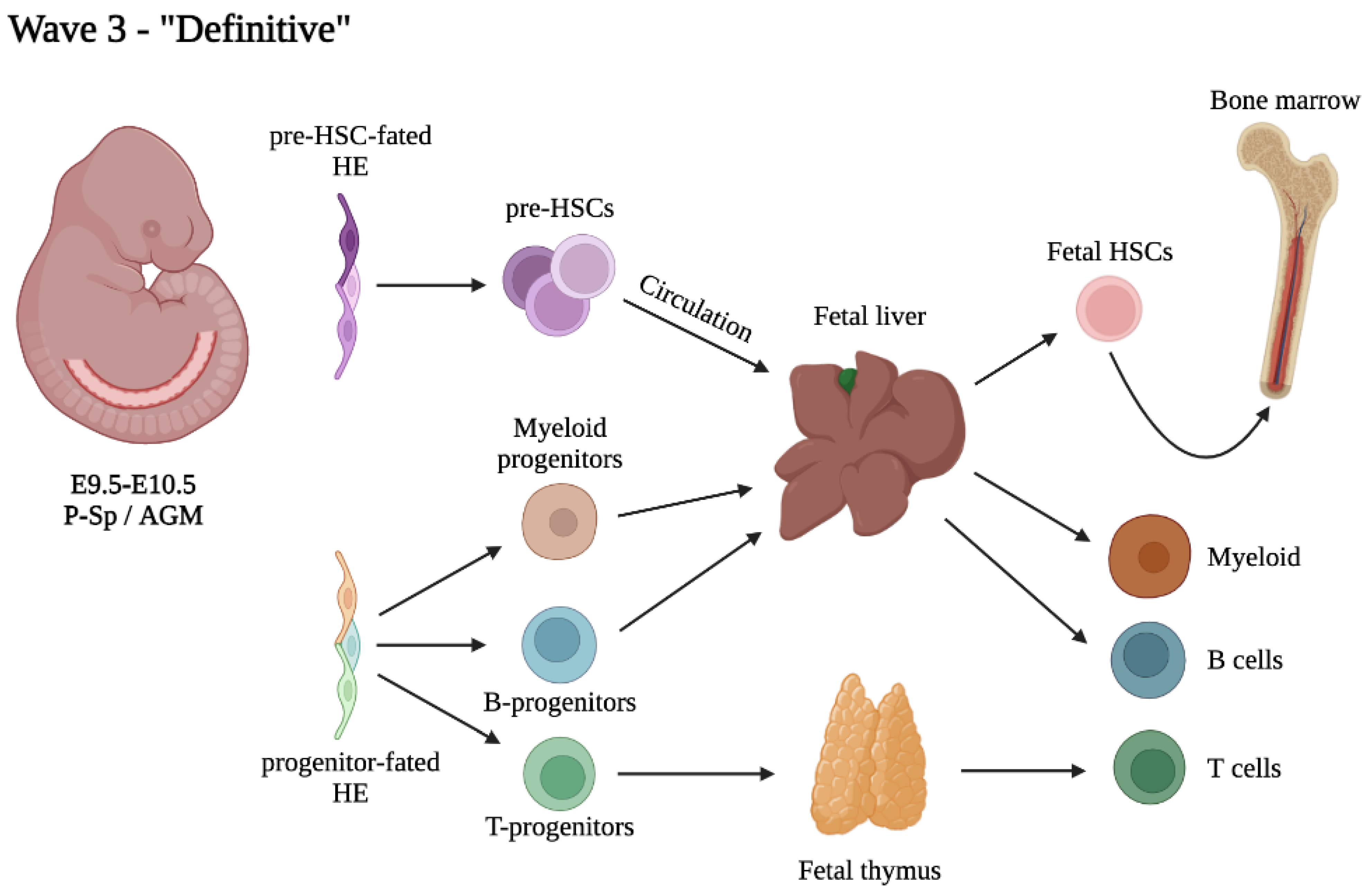
4. The Third and Definitive Wave: Not Just HSCs
4.1. Heterogeneity within the Pool of Intra-Aortic Clusters
4.2. Heterogeneity of Embryonic and Fetal HSCs
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, P.D.F. The development in vitro of the blood of the early chick embryo. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1932, 111, 497–521. [Google Scholar] [CrossRef]
- Choi, K.; Kennedy, M.; Kazarov, A.; Papadimitriou, J.C.; Keller, G. A common precursor for hematopoietic and endothelial cells. Development 1998, 125, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.L.; Kouskoff, V.; Fehling, H.J.; Palis, J.; Keller, G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 2004, 432, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Vogeli, K.M.; Jin, S.-W.; Martin, G.R.; Stainier, D.Y.R. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 2006, 443, 337–339. [Google Scholar] [CrossRef]
- Bollerot, K.; Pouget, C.; Jaffredo, T. The embryonic origins of hematopoietic stem cells: A tale of hemangioblast and hemogenic endothelium. APMIS 2005, 113, 790–803. [Google Scholar] [CrossRef]
- Lancrin, C.; Sroczynska, P.; Stephenson, C.; Allen, T.; Kouskoff, V.; Lacaud, G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009, 457, 892–895. [Google Scholar] [CrossRef]
- Padrón-Barthe, L.; Temiño, S.; del Campo, C.V.; Carramolino, L.; Isern, J.; Torres, M. Clonal analysis identifies hemogenic endothelium as the source of the blood-endothelial common lineage in the mouse embryo. Blood 2014, 124, 2523–2532. [Google Scholar] [CrossRef]
- Müller, A.M.; Medvinsky, A.; Strouboulis, J.; Grosveld, F.; Dzierzakt, E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1994, 1, 291–301. [Google Scholar] [CrossRef]
- Cumano, A.; Dieterlen-Lievre, F.; Godin, I. Lymphoid Potential, Probed before Circulation in Mouse, Is Restricted to Caudal Intraembryonic Splanchnopleura. Cell 1996, 86, 907–916. [Google Scholar] [CrossRef]
- Garcia-Porrero, J.A.; Godin, I.E.; Dieterlen-Lièvre, F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. 1995, 192, 425–435. [Google Scholar] [CrossRef]
- Godin, I.E.; Garcia-Porrero, J.A.; Coutinho, A.; Dieterlen-Lièvre, F.; Marcos, M.A.R. Fran Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 1993, 364, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Medvinsky, A.; Dzierzak, E. Definitive Hematopoiesis Is Autonomously Initiated by the AGM Region. Cell 1996, 86, 897–906. [Google Scholar] [CrossRef]
- Medvinsky, A.L.; Samoylina, N.L.; Müller, A.M.; Dzierzak, E.A.; M, A.M. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature 1993, 364, 64–67. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, M.F.; Speck, N.A.; Peeters, M.C.; Dzierzak, E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000, 19, 2465–2474. [Google Scholar] [CrossRef]
- Rybtsov, S.; Sobiesiak, M.; Taoudi, S.; Souilhol, C.; Senserrich, J.; Liakhovitskaia, A.; Ivanovs, A.; Frampton, J.; Zhao, S.; Medvinsky, A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 2011, 208, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Rybtsov, S.; Batsivari, A.; Bilotkach, K.; Paruzina, D.; Senserrich, J.; Nerushev, O.; Medvinsky, A. Tracing the Origin of the HSC Hierarchy Reveals an SCF-Dependent, IL-3-Independent CD43− Embryonic Precursor. Stem Cell Rep. 2014, 3, 489–501. [Google Scholar] [CrossRef]
- Yokomizo, T.; Dzierzak, E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 2010, 137, 3651–3661. [Google Scholar] [CrossRef]
- Taoudi, S.; Gonneau, C.; Moore, K.; Sheridan, J.M.; Blackburn, C.C.; Taylor, E.; Medvinsky, A. Extensive Hematopoietic Stem Cell Generation in the AGM Region via Maturation of VE-Cadherin+CD45+ Pre-Definitive HSCs. Cell Stem Cell 2008, 3, 99–108. [Google Scholar] [CrossRef]
- Gordon-Keylock, S.; Sobiesiak, M.; Rybtsov, S.; Moore, K.; Medvinsky, A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood 2013, 122, 2338–2345. [Google Scholar] [CrossRef]
- Swiers, G.; Rode, C.; Azzoni, E.; de Bruijn, M.F. A short history of hemogenic endothelium. Blood Cells Mol. Dis. 2013, 51, 206–212. [Google Scholar] [CrossRef]
- Jaffredo, T.; Gautier, R.; Eichmann, A.; Dieterlen-Lièvre, F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 1998, 125, 4575–4583. [Google Scholar] [CrossRef] [PubMed]
- Zovein, A.C.; Hofmann, J.J.; Lynch, M.; French, W.J.; Turlo, K.A.; Yang, Y.; Becker, M.S.; Zanetta, L.; Dejana, E.; Gasson, J.C.; et al. Fate Tracing Reveals the Endothelial Origin of Hematopoietic Stem Cells. Cell Stem Cell 2008, 3, 625–636. [Google Scholar] [CrossRef]
- Eilken, H.M.; Nishikawa, S.-I.; Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 2009, 457, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.; Traver, D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Boisset, J.-C.; Van Cappellen, W.; Andrieu-Soler, C.; Galjart, N.; Dzierzak, E.; Robin, C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 2010, 464, 116–120. [Google Scholar] [CrossRef]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef]
- Ditadi, A.; Sturgeon, C.M.; Tober, J.; Awong, G.; Kennedy, M.; Yzaguirre, A.D.; Azzola, L.; Ng, E.S.; Stanley, E.G.; French, D.L.; et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat. Cell Biol. 2015, 17, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Swiers, G.; Baumann, C.; O’Rourke, J.; Giannoulatou, E.; Taylor, S.; Joshi, A.; Moignard, V.; Pina, C.; Bee, T.; Kokkaliaris, K.D.; et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat. Commun. 2013, 4, 2924. [Google Scholar] [CrossRef]
- Ottersbach, K. Endothelial-to-haematopoietic transition: An update on the process of making blood. Biochem. Soc. Trans. 2019, 47, 591–601. [Google Scholar] [CrossRef]
- Yzaguirre, A.D.; Speck, N.A. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev. Dyn. 2016, 245, 1011–1028. [Google Scholar] [CrossRef]
- Frame, J.M.; Fegan, K.H.; Conway, S.J.; McGrath, K.E.; Palis, J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells 2015, 34, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Yvernogeau, L.; Gautier, R.; Petit, L.; Khoury, H.; Relaix, F.; Ribes, V.; Sang, H.; Charbord, P.; Souyri, M.; Robin, C.; et al. In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat. Cell Biol. 2019, 21, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Azzoni, E.; Conti, V.; Campana, L.; Dellavalle, A.; Adams, R.H.; Cossu, G.; Brunelli, S. Hemogenic endothelium generates mesoangioblasts that contribute to several mesodermal lineages in vivo. Development 2014, 141, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, R.; Jha, D.K.; Han, A.; Soria-Valles, C.; da Rocha, E.L.; Lu, Y.-F.; Goettel, J.A.; Serrao, E.; Rowe, R.G.; Malleshaiah, M.; et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017, 545, 432–438. [Google Scholar] [CrossRef]
- Lis, R.; Karrasch, C.C.; Poulos, M.G.; Kunar, B.; Redmond, D.; Duran, J.G.B.; Badwe, C.R.; Schachterle, W.; Ginsberg, M.; Xiang, J.; et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature 2017, 545, 439–445. [Google Scholar] [CrossRef]
- Ganuza, M.; Hadland, B.; Chabot, A.; Li, C.; Kang, G.; Bernstein, I.; McKinney-Freeman, S. Murine hemogenic endothelial precursors display heterogeneous hematopoietic potential ex vivo. Exp. Hematol. 2017, 51, 25–35.e6. [Google Scholar] [CrossRef]
- Chen, M.J.; Li, Y.; De Obaldia, M.E.; Yang, Q.; Yzaguirre, A.D.; Yamada-Inagawa, T.; Vink, C.S.; Bhandoola, A.; Dzierzak, E.; Speck, N.A. Erythroid/Myeloid Progenitors and Hematopoietic Stem Cells Originate from Distinct Populations of Endothelial Cells. Cell Stem Cell 2011, 9, 541–552. [Google Scholar] [CrossRef]
- Dignum, T.; Varnum-Finney, B.; Srivatsan, S.R.; Dozono, S.; Waltner, O.; Heck, A.M.; Ishida, T.; Nourigat-McKay, C.; Jackson, D.L.; Rafii, S.; et al. Multipotent progenitors and hematopoietic stem cells arise independently from hemogenic endothelium in the mouse embryo. Cell Rep. 2021, 36, 109675. [Google Scholar] [CrossRef]
- Vink, C.S.; Calero-Nieto, F.J.; Wang, X.; Maglitto, A.; Mariani, S.A.; Jawaid, W.; Gottgens, B.; Dzierzak, E. Iterative Sin-gle-Cell Analyses Define the Transcriptome of the First Functional Hematopoietic Stem Cells. Cell Rep. 2020, 31, 107627. [Google Scholar] [CrossRef]
- Simic, M.; Manosalva, I.; Spinelli, L.; Gentek, R.; Shayan, R.R.; Siret, C.; Girard-Madoux, M.; Wang, S.; de Fabritus, L.; Verschoor, J.; et al. Distinct Waves from the Hemogenic Endothelium Give Rise to Layered Lymphoid Tissue Inducer Cell Ontogeny. Cell Rep. 2020, 32, 108004. [Google Scholar] [CrossRef]
- Werner, Y.; Mass, E.; Kumar, P.A.; Ulas, T.; Händlers, K.; Horne, A.; Klee, K.; Lupp, A.; Schütz, D.; Saaber, F.; et al. Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat. Neurosci. 2020, 23, 351–362. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Gong, Y.; Hou, S.; Huang, T.; Wang, H.; Liu, D.; Ni, Y.; Wang, C.; Wang, J.; Hou, J.; et al. Spatiotemporal and Functional Heterogeneity of Hematopoietic Stem Cell-Competent Hemogenic Endothelial Cells in Mouse Embryos. Front. Cell Dev. Biol. 2021, 9, 699263. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Ghorbanian, Y.; Wang, W.; Wang, Y.; Kim, Y.J.; Weissman, I.L.; Inlay, M.A.; Mikkola, H.K. LYVE1 Marks the Divergence of Yolk Sac Definitive Hemogenic Endothelium from the Primitive Erythroid Lineage. Cell Rep. 2016, 17, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Ganuza, M.; Chabot, A.; Tang, X.; Bi, W.; Natarajan, S.; Carter, R.; Gawad, C.; Kang, G.; Cheng, Y.; McKinney-Freeman, S. Murine hematopoietic stem cell activity is derived from pre-circulation embryos but not yolk sacs. Nat. Commun. 2018, 9, 5405. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Cisson, J.L.; Stachura, D.L.; Traver, D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood 2010, 115, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Hadland, B.K.; Huppert, S.S.; Kanungo, J.; Xue, Y.; Jiang, R.; Gridley, T.; Conlon, R.A.; Cheng, A.M.; Kopan, R.; Longmore, G.D. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 2004, 104, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Kumano, K.; Chiba, S.; Kunisato, A.; Sata, M.; Saito, T.; Nakagami-Yamaguchi, E.; Yamaguchi, T.; Masuda, S.; Shimizu, K.; Takahashi, T.; et al. Notch1 but Not Notch2 Is Essential for Generating Hematopoietic Stem Cells from Endothelial Cells. Immunity 2003, 18, 699–711. [Google Scholar] [CrossRef]
- Uenishi, G.I.; Jung, H.S.; Kumar, A.; Park, M.A.; Hadland, B.K.; McLeod, E.; Raymond, M.; Moskvin, O.; Zimmerman, C.E.; Theisen, D.J.; et al. NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells. Nat. Commun. 2018, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Bonkhofer, F.; Rispoli, R.; Pinheiro, P.; Krecsmarik, M.; Schneider-Swales, J.; Tsang, I.H.C.; De Bruijn, M.; Monteiro, R.; Peterkin, T.; Patient, R. Blood stem cell-forming haemogenic endothelium in zebrafish derives from arterial endothelium. Nat. Commun. 2019, 10, 3577. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Kumar, A.; Jung, H.S.; Uenishi, G.; Moskvin, O.V.; Thomson, J.A.; Slukvin, I.I. Activation of the Arterial Program Drives Development of Definitive Hemogenic Endothelium with Lymphoid Potential. Cell Rep. 2018, 23, 2467–2481. [Google Scholar] [CrossRef]
- North, T.E.; Goessling, W.; Peeters, M.; Li, P.; Ceol, C.; Lord, A.M.; Weber, G.J.; Harris, J.; Cutting, C.C.; Huang, P.; et al. Hematopoietic Stem Cell Development Is Dependent on Blood Flow. Cell 2009, 137, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.F.; Li, N.; Lee, H.J.; Adamo, L.; Evans, S.M.; Willey, H.E.; Arora, N.; Torisawa, Y.S.; Vickers, D.A.; Morris, S.A.; et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. J. Exp. Med. 2015, 212, 665–680. [Google Scholar] [CrossRef]
- Kim, P.G.; Nakano, H.; Das, P.P.; Chen, M.J.; Rowe, R.G.; Chou, S.S.; Ross, S.J.; Sakamoto, K.M.; Zon, L.I.; Schlaeger, T.M.; et al. Flow-induced protein kinase A–CREB pathway acts via BMP signaling to promote HSC emergence. J. Exp. Med. 2015, 212, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Lundin, V.; Sugden, W.W.; Theodore, L.N.; Sousa, P.M.; Han, A.; Chou, S.; Wrighton, P.J.; Cox, A.G.; Ingber, D.E.; Goessling, W.; et al. YAP Regulates Hematopoietic Stem Cell Formation in Response to the Biomechanical Forces of Blood Flow. Dev. Cell 2020, 52, 446–460.e5. [Google Scholar] [CrossRef]
- Azzoni, E.; Frontera, V.; Anselmi, G.; Rode, C.; James, C.; Deltcheva, E.M.; Demian, A.S.; Brown, J.; Barone, C.; Patelli, A.; et al. The onset of circulation triggers a metabolic switch required for endothelial to hematopoietic transition. Cell Rep. 2021, 37, 110103. [Google Scholar] [CrossRef] [PubMed]
- Lux, C.T.; Yoshimoto, M.; McGrath, K.; Conway, S.J.; Palis, J.; Yoder, M.C. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 2008, 111, 3435–3438. [Google Scholar] [CrossRef]
- Yokomizo, T.; Watanabe, N.; Umemoto, T.; Matsuo, J.; Harai, R.; Kihara, Y.; Nakamura, E.; Tada, N.; Sato, T.; Takaku, T.; et al. Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J. Exp. Med. 2019, 216, 1599–1614. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, P.; Tober, J.; Bennett, L.; Chen, C.; Uzun, Y.; Li, Y.; Howell, E.D.; Mumau, M.; Yu, W.; et al. Developmental trajectory of prehematopoietic stem cell formation from endothelium. Blood 2020, 136, 845–856. [Google Scholar] [CrossRef]
- Baron, C.S.; Kester, L.; Klaus, A.; Boisset, J.-C.; Thambyrajah, R.; Yvernogeau, L.; Kouskoff, V.; Lacaud, G.; Van Oudenaarden, A.; Robin, C. Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat. Commun. 2018, 9, 2517. [Google Scholar] [CrossRef]
- Fadlullah, M.Z.H.; Neo, W.H.; Lie-A-Ling, M.; Thambyrajah, R.; Patel, R.; Mevel, R.; Aksoy, I.; Khoa, N.D.; Savatier, P.; Fontenille, L.; et al. Murine AGM single-cell profiling identifies a continuum of hemogenic endothelium differentiation marked by ACE. Blood 2022, 139, 343–356. [Google Scholar] [CrossRef]
- Oatley, M.; Bölükbası, Ö.V.; Svensson, V.; Shvartsman, M.; Ganter, K.; Zirngibl, K.; Pavlovich, P.V.; Milchevskaya, V.; Foteva, V.; Natarajan, K.N.; et al. Single-cell transcriptomics identifies CD44 as a marker and regulator of endothelial to haematopoietic transition. Nat. Commun. 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Yvernogeau, L.; Klaus, A.; Maas, J.; Morin-Poulard, I.; Weijts, B.; Schulte-Merker, S.; Berezikov, E.; Junker, J.P.; Robin, C. Multispecies RNA tomography reveals regulators of hematopoietic stem cell birth in the embryonic aorta. Blood 2020, 136, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Weijts, B.; Yvernogeau, L.; Robin, C. Recent Advances in Developmental Hematopoiesis: Diving Deeper With New Technologies. Front. Immunol. 2021, 12, 790379. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Li, Z.; Zheng, X.; Gao, Y.; Dong, J.; Ni, Y.; Wang, X.; Li, Y.; Ding, X.; Chang, Z.; et al. Embryonic endothelial evolution towards first hematopoietic stem cells revealed by single-cell transcriptomic and functional analyses. Cell Res. 2020, 30, 376–392. [Google Scholar] [CrossRef]
- Crosse, E.I.; Gordon-Keylock, S.; Rybtsov, S.; Binagui-Casas, A.; Felchle, H.; Nnadi, N.C.; Kirschner, K.; Chandra, T.; Tamagno, S.; Webb, D.J.; et al. Multi-layered Spatial Transcriptomics Identify Secretory Factors Promoting Human Hematopoietic Stem Cell Development. Cell Stem Cell 2020, 27, 822–839.e8. [Google Scholar] [CrossRef]
- Zeng, Y.; He, J.; Bai, Z.; Li, Z.; Gong, Y.; Liu, C.; Ni, Y.; Du, J.; Ma, C.; Bian, L.; et al. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res. 2019, 29, 881–894. [Google Scholar] [CrossRef]
- Gao, L.; Tober, J.; Gao, P.; Chen, C.; Tan, K.; Speck, N.A. RUNX1 and the endothelial origin of blood. Exp. Hematol. 2018, 68, 2–9. [Google Scholar] [CrossRef]
- McGarvey, A.; Rybtsov, S.; Souilhol, C.; Tamagno, S.; Rice, R.; Hills, D.; Godwin, D.; Rice, D.; Tomlinson, S.R.; Medvinsky, A. A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation. J. Exp. Med. 2017, 214, 3731–3751. [Google Scholar] [CrossRef]
- Souilhol, C.; Gonneau, C.; Lendinez, J.G.; Batsivari, A.; Rybtsov, S.; Wilson, H.; Morgado-Palacin, L.; Hills, D.; Taoudi, S.; Antonchuk, J.; et al. Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat. Commun. 2016, 7, 10784. [Google Scholar] [CrossRef]
- Azzoni, E.; Frontera, V.; E McGrath, K.; Harman, J.; Carrelha, J.; Nerlov, C.; Palis, J.; Jacobsen, S.E.W.; De Bruijn, M.F. Kit ligand has a critical role in mouse yolk sac and aorta–gonad–mesonephros hematopoiesis. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef]
- Mirshekar-Syahkal, B.; Fitch, S.R.; Ottersbach, K. Concise Review: From Greenhouse to Garden: The Changing Soil of the Hematopoietic Stem Cell Microenvironment During Development. Stem Cells 2014, 32, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Soares-Da-Silva, F.; Freyer, L.; Elsaid, R.; Burlen-Defranoux, O.; Iturri, L.; Sismeiro, O.; Pinto-Do-Ó, P.; Gomez-Perdiguero, E.; Cumano, A. Yolk sac, but not hematopoietic stem cell–derived progenitors, sustain erythropoiesis throughout murine embryonic life. J. Exp. Med. 2021, 218, 218. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.H.; Lie-A-Ling, M.; Fadlullah, M.Z.H.; Lacaud, G. Contributions of Embryonic HSC-Independent Hematopoiesis to Organogenesis and the Adult Hematopoietic System. Front. Cell Dev. Biol. 2021, 9, 631699. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999, 126, 5073–5084. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Hematopoietic stem cell-independent hematopoiesis: Emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. FEBS Lett. 2016, 590, 3965–3974. [Google Scholar] [CrossRef]
- Okuda, T.; van Deursen, J.; Hiebert, S.W.; Grosveld, G.; Downing, J.R. AML1, the Target of Multiple Chromosomal Translocations in Human Leukemia, Is Essential for Normal Fetal Liver Hematopoiesis. Cell 1996, 84, 321–330. [Google Scholar] [CrossRef]
- Wittamer, V.; Bertrand, J.Y. Yolk sac hematopoiesis: Does it contribute to the adult hematopoietic system? Cell. Mol. Life Sci. 2020, 77, 4081–4091. [Google Scholar] [CrossRef]
- McGrath, K.E.; Frame, J.M.; Fegan, K.H.; Bowen, J.R.; Conway, S.J.; Catherman, S.C.; Kingsley, P.D.; Koniski, A.D.; Palis, J. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep. 2015, 11, 1892–1904. [Google Scholar] [CrossRef]
- Böiers, C.; Carrelha, J.; Lutteropp, M.; Luc, S.; Green, J.C.; Azzoni, E.; Woll, P.S.; Mead, A.J.; Hultquist, A.; Swiers, G.; et al. Lymphomyeloid Contribution of an Immune-Restricted Progenitor Emerging Prior to Definitive Hematopoietic Stem Cells. Cell Stem Cell 2013, 13, 535–548. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Montecino-Rodriguez, E.; Ferkowicz, M.J.; Porayette, P.; Shelley, W.C.; Conway, S.J.; Dorshkind, K.; Yoder, M.C. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl. Acad. Sci. USA 2011, 108, 1468–1473. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Porayette, P.; Glosson, N.L.; Conway, S.J.; Carlesso, N.; Cardoso, A.A.; Kaplan, M.H.; Yoder, M.C. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 2012, 119, 5706–5714. [Google Scholar] [CrossRef] [PubMed]
- Hadland, B.; Yoshimoto, M. Many layers of embryonic hematopoiesis: New insights into B-cell ontogeny and the origin of hematopoietic stem cells. Exp. Hematol. 2018, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rybtsov, S.; Ivanovs, A.; Zhao, S.; Medvinsky, A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 2016, 143, 1284–1289. [Google Scholar] [CrossRef]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.D.; Malik, J.; Emerson, R.L.; Bushnell, T.P.; McGrath, K.E.; Bloedorn, L.A.; Bulger, M.; Palis, J. “Maturational” globin switching in primary primitive erythroid cells. Blood 2006, 107, 1665–1672. [Google Scholar] [CrossRef]
- Kingsley, P.D.; Malik, J.; Fantauzzo, K.A.; Palis, J. Yolk sac–derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 2004, 104, 19–25. [Google Scholar] [CrossRef]
- Garcia-Alegria, E.; Menegatti, S.; Fadlullah, M.Z.; Menendez, P.; Lacaud, G.; Kouskoff, V. Early Human Hemogenic Endothelium Generates Primitive and Definitive Hematopoiesis In Vitro. Stem Cell Rep. 2018, 11, 1061–1074. [Google Scholar] [CrossRef]
- Stefańska, M.; Batta, K.; Patel, R.; Florkowska, M.; Kouskoff, V.; Lacaud, G. Primitive erythrocytes are generated from hemogenic endothelial cells. Sci. Rep. 2017, 7, 6401. [Google Scholar] [CrossRef]
- Lacaud, G.; Gore, L.; Kennedy, M.; Kouskoff, V.; Kingsley, P.; Hogan, C.; Carlsson, L.; Speck, N.; Palis, J.; Keller, G. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 2002, 100, 458–466. [Google Scholar] [CrossRef]
- Yokomizo, T.; Hasegawa, K.; Ishitobi, H.; Osato, M.; Ema, M.; Ito, Y.; Yamamoto, M.; Takahashi, S. Runx1 is involved in primitive erythropoiesis in the mouse. Blood 2008, 111, 4075–4080. [Google Scholar] [CrossRef]
- Tober, J.; Koniski, A.; McGrath, K.E.; Vemishetti, R.; Emerson, R.; De Mesy-Bentley, K.K.L.; Waugh, R.; Palis, J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 2006, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Potts, K.S.; Sargeant, T.J.; Markham, J.F.; Shi, W.; Biben, C.; Josefsson, E.C.; Whitehead, L.W.; Rogers, K.L.; Liakhovitskaia, A.; Smyth, G.K.; et al. A lineage of diploid platelet-forming cells precedes polyploid megakaryocyte formation in the mouse embryo. Blood 2014, 124, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Iturri, L.; Freyer, L.; Biton, A.; Dardenne, P.; Lallemand, Y.; Perdiguero, E.G. Megakaryocyte production is sustained by direct differentiation from erythromyeloid progenitors in the yolk sac until midgestation. Immunity 2021, 54, 1433–1446.e5. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Jalil, A.; Klaine, M.; Jung, S.; Cumano, A.; Godin, I. Three pathways to mature macrophages in the early mouse yolk sac. Blood 2005, 106, 3004–3011. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.J.; Stacy, T.; Speck, N.A. Runx1 function in hematopoiesis is required in cells that express Tek. Blood 2006, 107, 106–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef]
- Ovchinnikov, D.A. Macrophages in the embryo and beyond: Much more than just giant phagocytes. Genesis 2008, 46, 447–462. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- McGrath, K.E.; Frame, J.M.; Palis, J. Early hematopoiesis and macrophage development. Semin. Immunol. 2015, 27, 379–387. [Google Scholar] [CrossRef]
- Ferrero, G.; Mahony, C.; Dupuis, E.; Yvernogeau, L.; Di Ruggiero, E.; Miserocchi, M.; Caron, M.; Robin, C.; Traver, D.; Bertrand, J.Y.; et al. Embryonic Microglia Derive from Primitive Macrophages and Are Replaced by cmyb-Dependent Definitive Microglia in Zebrafish. Cell Rep. 2018, 24, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef]
- Godin, I.; Dieterlen-Lièvre, F.; Cumano, A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc. Natl. Acad. Sci. USA 1995, 92, 773–777. [Google Scholar] [CrossRef]
- Wong, P.M.; Chung, S.W.; Reicheld, S.M.; Chui, D.H. Hemoglobin switching during murine embryonic development: Evidence for two populations of embryonic erythropoietic progenitor cells. Blood 1986, 67, 716–721. [Google Scholar] [CrossRef]
- Palis, J.; Chan, R.J.; Koniski, A.; Patel, R.; Starr, M.; Yoder, M.C. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 4528–4533. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Giroux, S.; Golub, R.; Klaine, M.; Jalil, A.; Boucontet, L.; Godin, I.; Cumano, A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc. Natl. Acad. Sci. USA 2004, 102, 134–139. [Google Scholar] [CrossRef]
- Dzierzak, E.; Bigas, A. Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell 2018, 22, 639–651. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Pokrovskii, M.; Vicario, R.; Geissmann, F. Origins, Biology, and Diseases of Tissue Macrophages. Annu. Rev. Immunol. 2021, 39, 313–344. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.E.; Percin, G.I.; Muller, J.T.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.K.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019, 568, 541–545. [Google Scholar] [CrossRef]
- Yahara, Y.; Barrientos, T.; Tang, Y.J.; Puviindran, V.; Nadesan, P.; Zhang, H.; Gibson, J.R.; Gregory, S.G.; Diao, Y.; Xiang, Y.; et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020, 22, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Günther, P.; Crozet, L.; Jacome-Galarza, C.E.; Händler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of tissue-resident macrophages during organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.; Bennett, M.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Gephart, M.H.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Sakai, M.; Troutman, T.D.; Seidman, J.S.; Ouyang, Z.; Spann, N.J.; Abe, Y.; Ego, K.M.; Bruni, C.M.; Deng, Z.; Schlachetzki, J.C.; et al. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity 2019, 51, 655–670.e8. [Google Scholar] [CrossRef]
- Gray, J.I.; Farber, D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022, 40, 40. [Google Scholar] [CrossRef]
- Dege, C.; Fegan, K.H.; Creamer, J.P.; Berrien-Elliott, M.; Luff, S.A.; Kim, D.; Wagner, J.A.; Kingsley, P.D.; McGrath, K.E.; Fehniger, T.A.; et al. Potently Cytotoxic Natural Killer Cells Initially Emerge from Erythro-Myeloid Progenitors during Mammalian Development. Dev. Cell 2020, 53, 229–239.e7. [Google Scholar] [CrossRef]
- Mass, E.; Jacome-Galarza, C.E.; Blank, T.; Lazarov, T.; Durham, B.; Ozkaya, N.; Pastore, A.; Schwabenland, M.; Chung, Y.R.; Rosenblum, M.K.; et al. A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature 2017, 549, 389–393. [Google Scholar] [CrossRef]
- Fennie, C.; Cheng, J.; Dowbenko, D.; Young, P.; Lasky, L. CD34+ endothelial cell lines derived from murine yolk sac induce the proliferation and differentiation of yolk sac CD34+ hematopoietic progenitors. Blood 1995, 86, 4454–4467. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Auerbach, R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development 1991, 113, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zettergren, L.D.; Auerbach, R. In vitro differentiation of B cells and myeloid cells from the early mouse embryo and its extraembryonic yolk sac. Exp. Hematol. 1994, 22, 19–25. [Google Scholar] [PubMed]
- Kobayashi, M.; Shelley, W.C.; Seo, W.; Vemula, S.; Lin, Y.; Liu, Y.; Kapur, R.; Taniuchi, I.; Yoshimoto, M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc. Natl. Acad. Sci. USA 2014, 111, 12151–12156. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, E.E.B.; Waters, J.; Phillips, M.; Yamamoto, R.; Long, B.R.; Yang, Y.; Gerstein, R.; Stoddart, C.A.; Nakauchi, H.; Herzenberg, L.A. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Rep. 2016, 6, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, E.E.B.; Yamamoto, R.; Hamanaka, S.; Yang, Y.; Herzenberg, L.A.; Nakauchi, H. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 5394–5398. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Jorquera, A.; Msallam, R.; Wienert, S.; Klauschen, F.; Ginhoux, F.; Bajenoff, M. Epidermal gammadelta T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 2018, 215, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McGrath, K.E.; Ayoub, E.; Kingsley, P.D.; Yu, H.; Fegan, K.; McGlynn, K.A.; Rudzinskas, S.; Palis, J.; Perkins, A.S. Mds1, an inducible Cre allele specific to adult-repopulating hematopoietic stem cells. Cell Rep. 2021, 36, 109562. [Google Scholar] [CrossRef]
- Luis, T.C.; Luc, S.; Mizukami, T.; Boukarabila, H.; Thongjuea, S.; Woll, P.S.; Azzoni, E.; Giustacchini, A.; Lutteropp, M.; Bouriez-Jones, T.; et al. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nat. Immunol. 2016, 17, 1424–1435. [Google Scholar] [CrossRef]
- Elsaid, R.; Meunier, S.; Burlen-Defranoux, O.; Soares-Da-Silva, F.; Perchet, T.; Iturri, L.; Freyer, L.; Vieira, P.; Pereira, P.; Golub, R.; et al. A wave of bipotent T/ILC-restricted progenitors shapes the embryonic thymus microenvironment in a time-dependent manner. Blood 2021, 137, 1024–1036. [Google Scholar] [CrossRef]
- de Bruijn, M.F.; Ma, X.; Robin, C.; Ottersbach, K.; Sánchez, M.J.; Dzierzak, E. Hematopoietic Stem Cells Localize to the Endothelial Cell Layer in the Midgestation Mouse Aorta. Immunity 2002, 16, 673–683. [Google Scholar] [CrossRef]
- Boisset, J.-C.; Clapes, T.; Klaus, A.; Papazian, N.; Onderwater, J.; Mommaas-Kienhuis, M.; Cupedo, T.; Robin, C. Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood 2015, 125, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Taoudi, S.; Medvinsky, A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc. Natl. Acad. Sci. USA 2007, 104, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Hadland, B.; Tan, K.; Speck, N.A. CD27 marks murine embryonic hematopoietic stem cells and type II prehematopoietic stem cells. Blood 2017, 130, 372–376. [Google Scholar] [CrossRef]
- Tang, W.; He, J.; Huang, T.; Bai, Z.; Wang, C.; Wang, H.; Yang, R.; Ni, Y.; Hou, J.; Wang, J.; et al. Hlf Expression Marks Early Emergence of Hematopoietic Stem Cell Precursors With Adult Repopulating Potential and Fate. Front. Cell Dev. Biol. 2021, 9, 728057. [Google Scholar] [CrossRef]
- Zhou, F.; Li, X.; Wang, W.; Zhu, P.; Zhou, J.; He, W.; Ding, M.; Xiong, F.; Zheng, X.; Li, Z.; et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature 2016, 533, 487–492. [Google Scholar] [CrossRef]
- de Pater, E.; Kaimakis, P.; Vink, C.S.; Yokomizo, T.; Yamada-Inagawa, T.; van der Linden, R.; Kartalaei, P.S.; Camper, S.A.; Speck, N.; Dzierzak, E. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013, 210, 2843–2850. [Google Scholar] [CrossRef]
- Carrelha, J.; Meng, Y.; Kettyle, L.M.; Luis, T.C.; Norfo, R.; Alcolea, V.; Boukarabila, H.; Grasso, F.; Gambardella, A.; Grover, A.; et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature 2018, 554, 106–111. [Google Scholar] [CrossRef]
- Rodriguez-Fraticelli, A.E.; Weinreb, C.; Wang, S.-W.; Migueles, R.P.; Jankovic, M.; Usart, M.; Klein, A.M.; Lowell, S.; Camargo, F.D. Single-cell lineage tracing unveils a role for TCF15 in haematopoiesis. Nature 2020, 583, 585–589. [Google Scholar] [CrossRef]
- Rodriguez-Fraticelli, A.E.; Camargo, F. Systems analysis of hematopoiesis using single-cell lineage tracing. Curr. Opin. Hematol. 2021, 28, 18–27. [Google Scholar] [CrossRef]
- Oguro, H.; Ding, L.; Morrison, S.J. SLAM Family Markers Resolve Functionally Distinct Subpopulations of Hematopoietic Stem Cells and Multipotent Progenitors. Cell Stem Cell 2013, 13, 102–116. [Google Scholar] [CrossRef]
- Crisan, M.; Dzierzak, E. The many faces of hematopoietic stem cell heterogeneity. Development 2016, 143, 4571–4581. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Trumpp, A.; Milsom, M.D. Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell Stem Cell 2018, 22, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Jurecic, R. Hematopoietic Stem Cell Heterogeneity. Adv. Exp. Med. Biol. 2019, 1169, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Epah, J.; Schafer, R. Implications of hematopoietic stem cells heterogeneity for gene therapies. Gene Ther. 2021, 28, 528–541. [Google Scholar] [CrossRef]
- Pei, W.; Feyerabend, T.B.; Rössler, J.; Wang, X.; Postrach, D.; Busch, K.; Rode, I.; Klapproth, K.; Dietlein, N.; Quedenau, C.; et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 2017, 548, 456–460. [Google Scholar] [CrossRef]
- Bowling, S.; Sritharan, D.; Osorio, F.G.; Nguyen, M.; Cheung, P.; Rodriguez-Fraticelli, A.; Patel, S.; Yuan, W.C.; Fujiwara, Y.; Li, B.E.; et al. An Engineered CRISPR-Cas9 Mouse Line for Simultaneous Readout of Lineage Histories and Gene Expression Profiles in Single Cells. Cell 2020, 181, 1410–1422.e27. [Google Scholar] [CrossRef]
- Pei, W.; Shang, F.; Wang, X.; Fanti, A.-K.; Greco, A.; Busch, K.; Klapproth, K.; Zhang, Q.; Quedenau, C.; Sauer, S.; et al. Resolving Fates and Single-Cell Transcriptomes of Hematopoietic Stem Cell Clones by PolyloxExpress Barcoding. Cell Stem Cell 2020, 27, 383–395.e8. [Google Scholar] [CrossRef]
- Ottersbach, K.; P, K.; L, H.; Am, M.; J, U.; S, Z.; S, Z.; J, A.; A, M. Faculty Opinions recommendation of Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): Role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2016, 129, 4891–4899. [Google Scholar] [CrossRef]
- Crisan, M.; Kartalaei, P.S.; Vink, C.S.; Yamada-Inagawa, T.; Bollerot, K.; Van Ijcken, W.; van der Linden, R.; Lopes, S.M.C.D.S.; Monteiro, R.; Mummery, C.; et al. BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat. Commun. 2015, 6, 8040. [Google Scholar] [CrossRef]
- Crisan, M.; Kartalaei, P.S.; Neagu, A.; Karkanpouna, S.; Yamada-Inagawa, T.; Purini, C.; Vink, C.S.; van der Linden, R.; van Ijcken, W.; Lopes, S.M.C.D.S.; et al. BMP and Hedgehog Regulate Distinct AGM Hematopoietic Stem Cells Ex Vivo. Stem Cell Rep. 2016, 6, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Copley, M.R.; Eaves, C.J. Developmental changes in hematopoietic stem cell properties. Exp. Mol. Med. 2013, 45, e55. [Google Scholar] [CrossRef] [PubMed]
- Bowie, M.B.; Kent, D.G.; Copley, M.R.; Eaves, C.J. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood 2007, 109, 5043–5048. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Boyer, S.W.; Perez-Cunningham, J.; Hernandez, G.E.; Derderian, S.C.; Jujjavarapu, C.; Aaserude, E.; MacKenzie, T.; Forsberg, E.C. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell 2016, 19, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Bowie, M.B.; Kent, D.G.; Dykstra, B.; McKnight, K.D.; McCaffrey, L.; Hoodless, P.A.; Eaves, C.J. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl. Acad. Sci. USA 2007, 104, 5878–5882. [Google Scholar] [CrossRef]
- Li, Y.; Kong, W.; Yang, W.; Patel, R.M.; Casey, E.B.; Okeyo-Owuor, T.; White, J.M.; Porter, S.N.; Morris, S.A.; Magee, J.A. Single-Cell Analysis of Neonatal HSC Ontogeny Reveals Gradual and Uncoordinated Transcriptional Reprogramming that Begins before Birth. Cell Stem Cell 2020, 27, 732–747.e7. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, G.; Sommarin, M.N.E.; Böiers, C. Defining the Emerging Blood System During Development at Single-Cell Resolution. Front. Cell Dev. Biol. 2021, 9, 660350. [Google Scholar] [CrossRef]
- Gensollen, T.; Lin, X.; Zhang, T.; Pyzik, M.; See, P.; Glickman, J.N.; Ginhoux, F.; Waldor, M.; Salmi, M.; Rantakari, P.; et al. Embryonic macrophages function during early life to determine invariant natural killer T cell levels at barrier surfaces. Nat. Immunol. 2021, 22, 699–710. [Google Scholar] [CrossRef]
- Apostol, A.C.; Jensen, K.D.C.; Beaudin, A.E. Training the Fetal Immune System Through Maternal Inflammation—A Layered Hygiene Hypothesis. Front. Immunol. 2020, 11, 123. [Google Scholar] [CrossRef]
- Lopez, C.; Noguera, E.; Stavropoulou, V.; Robert, E.; Aid, Z.; Ballerini, P.; Bilhou-Nabera, C.; Lapillonne, H.; Boudia, F.; Thirant, C.; et al. Ontogenic Changes in Hematopoietic Hierarchy Determine Pediatric Specificity and Disease Phenotype in Fusion Oncogene-Driven Myeloid Leukemia. Cancer Discov. 2019, 9, 1736–1753. [Google Scholar] [CrossRef]
- Porter, S.N.; Cluster, A.S.; Yang, W.; A Busken, K.; Patel, R.M.; Ryoo, J.; A Magee, J. Fetal and neonatal hematopoietic progenitors are functionally and transcriptionally resistant to Flt3-ITD mutations. eLife 2016, 5, e18882. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cardoso, A.A.; Yoshimoto, M.; Kobayashi, M. The Earliest T-Precursors in the Mouse Embryo Are Susceptible to Leukemic Transformation. Front. Cell Dev. Biol. 2021, 9, 634151. [Google Scholar] [CrossRef] [PubMed]
- Gerr, H.; Zimmermann, M.; Schrappe, M.; Dworzak, M.; Ludwig, W.-D.; Bradtke, J.; Moericke, A.; Schabath, R.; Creutzig, U.; Reinhardt, D. Acute leukaemias of ambiguous lineage in children: Characterization, prognosis and therapy recommendations. Br. J. Haematol. 2010, 149, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Böiers, C.; Richardson, S.E.; Laycock, E.; Zriwil, A.; Turati, V.A.; Brown, J.; Wray, J.P.; Wang, D.; James, C.; Herrero, J.; et al. A Human IPS Model Implicates Embryonic B-Myeloid Fate Restriction as Developmental Susceptibility to B Acute Lymphoblastic Leukemia-Associated ETV6-RUNX. Dev. Cell 2018, 44, 362–377.e7. [Google Scholar] [CrossRef] [PubMed]

| HSC- and Lymphoid- Competent HE | EMP-Competent HE | References | |
|---|---|---|---|
| Notch pathway dependence |  |  | [45,46,47] |
| Arterial identity dependence |  (Lymphoid potential) (Lymphoid potential) |  | [48,49,50] |
| WNT canonical pathway dependence |  |  | [31] |
| Cxcr4 expression |  (Lymphoid potential) (Lymphoid potential) |  | [40] |
| Lyve1 expression | Low |  | [42,43,44] |
| Hlf expression |  |  (until E10.5) (until E10.5) | [57] |
| Blood flow dependence |  |  | [51,52,53,54,55,56] |
| Hypoxia/glycolysis | Decrease of hematopoietic output | Increase of hematopoietic output | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, C.; Orsenigo, R.; Meneveri, R.; Brunelli, S.; Azzoni, E. One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis. Cells 2022, 11, 1061. https://doi.org/10.3390/cells11061061
Barone C, Orsenigo R, Meneveri R, Brunelli S, Azzoni E. One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis. Cells. 2022; 11(6):1061. https://doi.org/10.3390/cells11061061
Chicago/Turabian StyleBarone, Cristiana, Roberto Orsenigo, Raffaella Meneveri, Silvia Brunelli, and Emanuele Azzoni. 2022. "One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis" Cells 11, no. 6: 1061. https://doi.org/10.3390/cells11061061
APA StyleBarone, C., Orsenigo, R., Meneveri, R., Brunelli, S., & Azzoni, E. (2022). One Size Does Not Fit All: Heterogeneity in Developmental Hematopoiesis. Cells, 11(6), 1061. https://doi.org/10.3390/cells11061061







