Artificial Oocyte: Development and Potential Application
Abstract
:1. Introduction
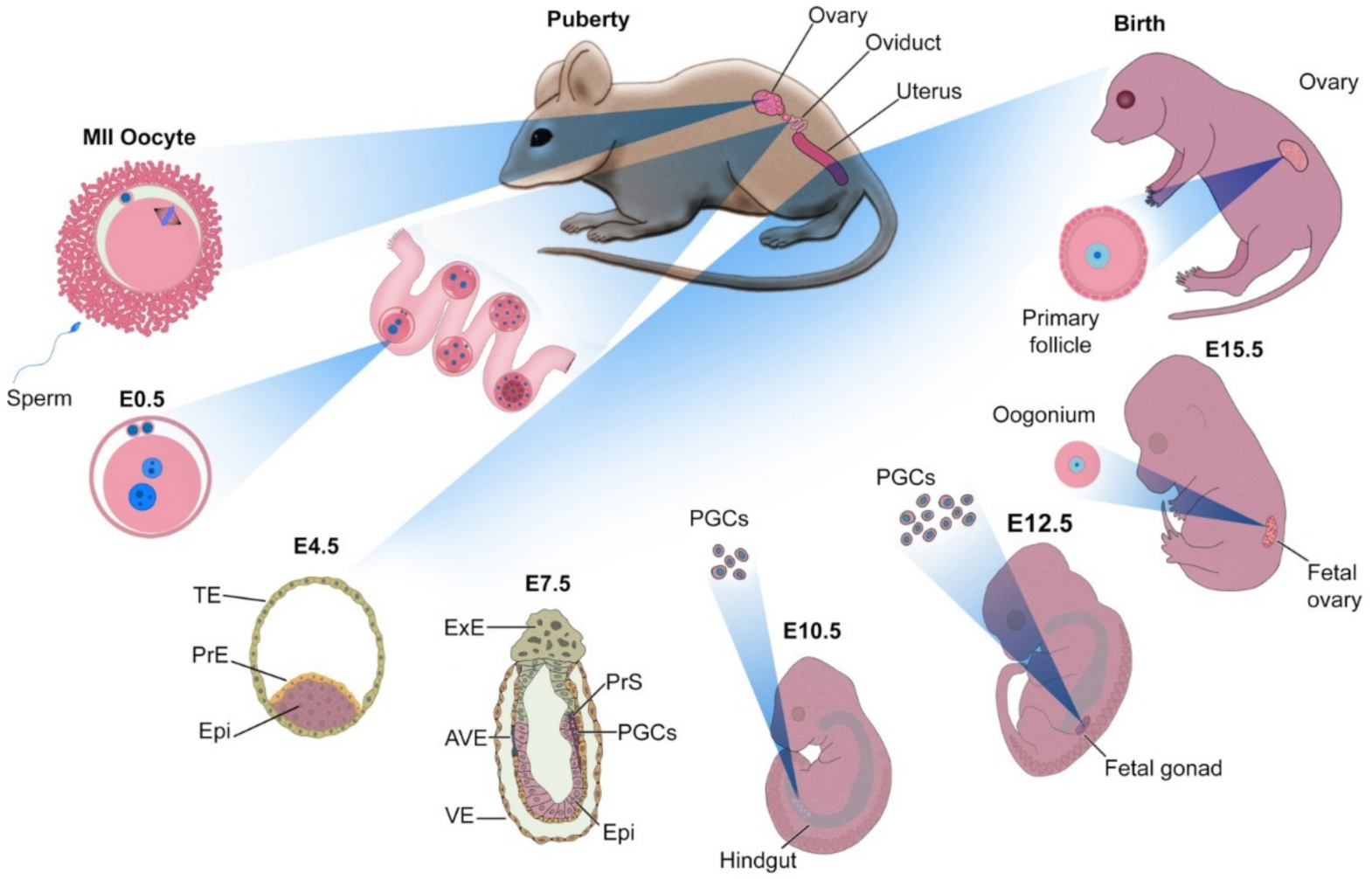
2. Principles of Oocyte Development
3. Sources of Oocytes
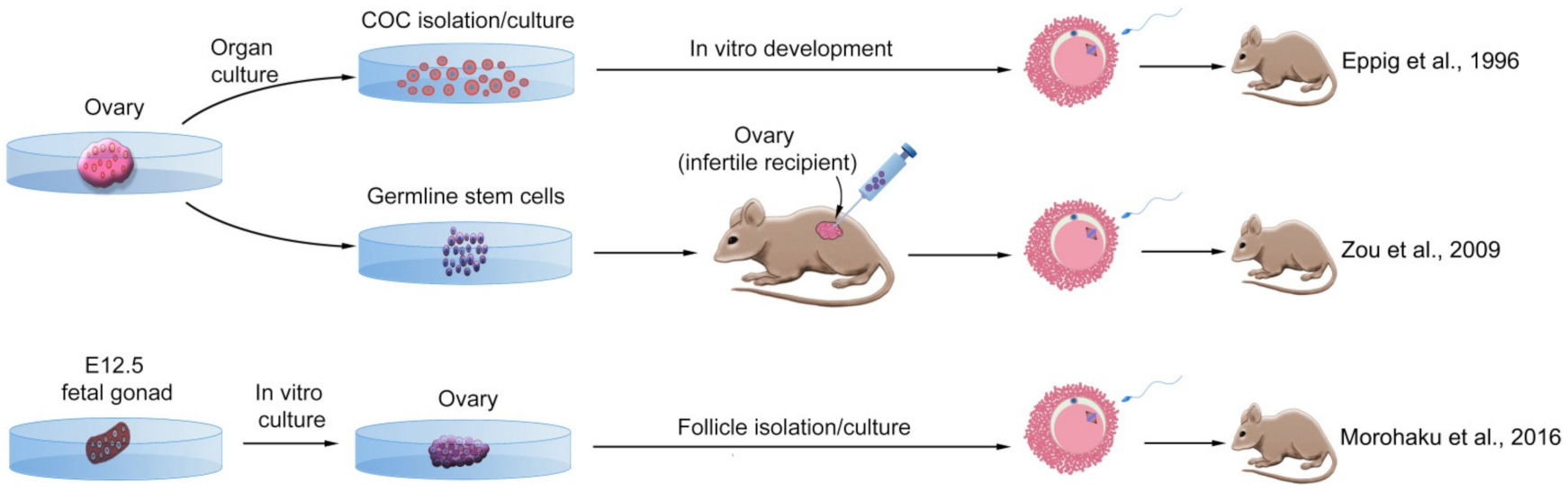
3.1. Maturation of Ovarian Follicles
3.1.1. Ovarian Follicle from Mouse
3.1.2. Ovarian Follicle from Human
3.2. Ovarian Stem Cells
3.2.1. Ovarian Stem Cells from Mouse
3.2.2. Ovarian Stem Cells from Human
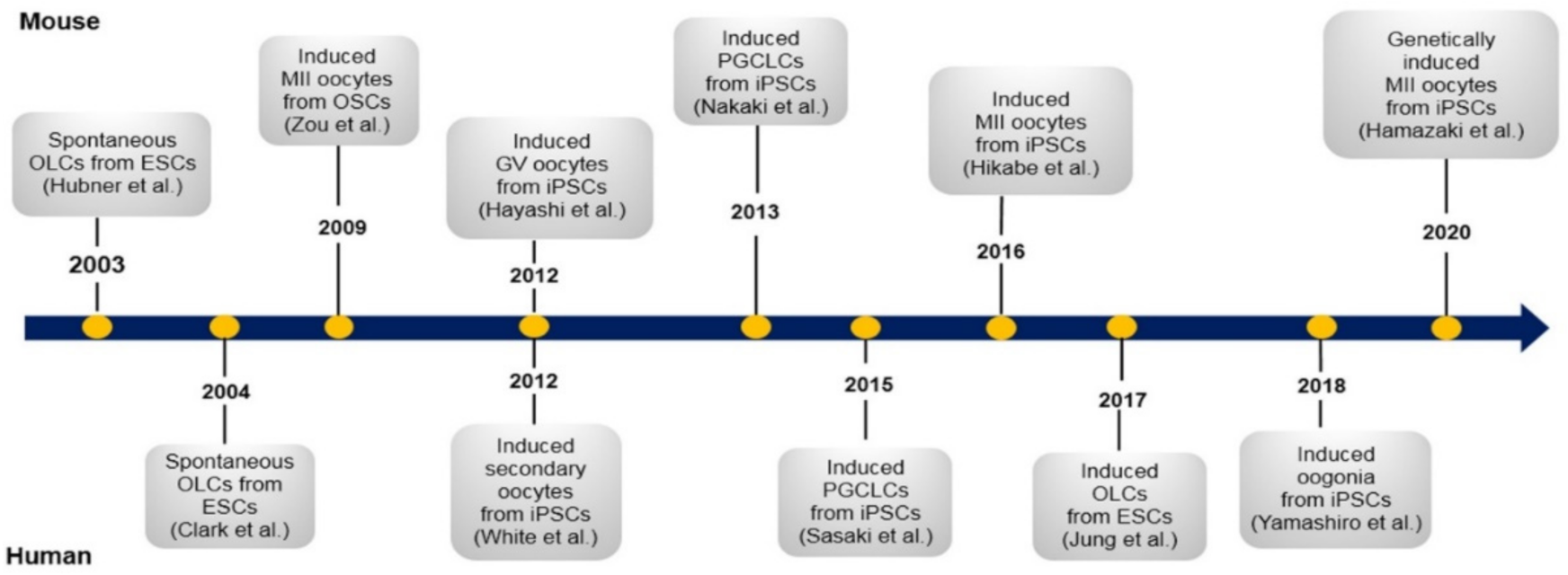
3.3. Pluripotent Stem Cells
3.3.1. Embryonic Stem Cells
Embryonic Stem Cells from Mouse
Embryonic Stem Cells from Human
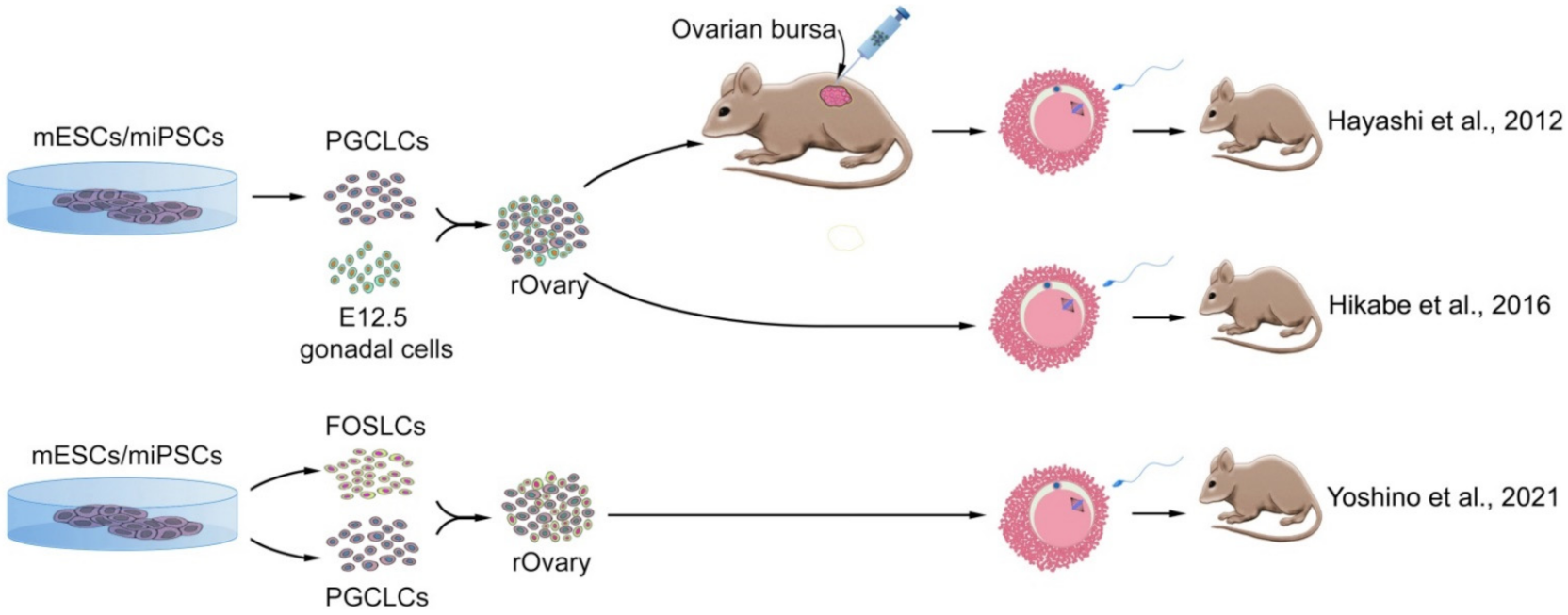
3.3.2. Induced Pluripotent Stem Cells
Induced Pluripotent Stem Cells from Mouse
Induced Pluripotent Stem Cells from Human
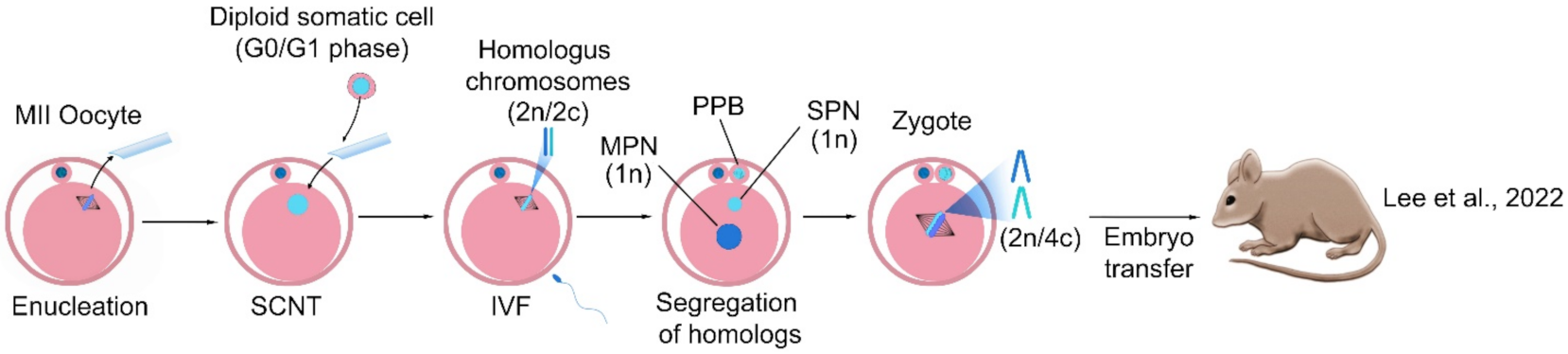
4. Haploidization in Somatic Chromosomes
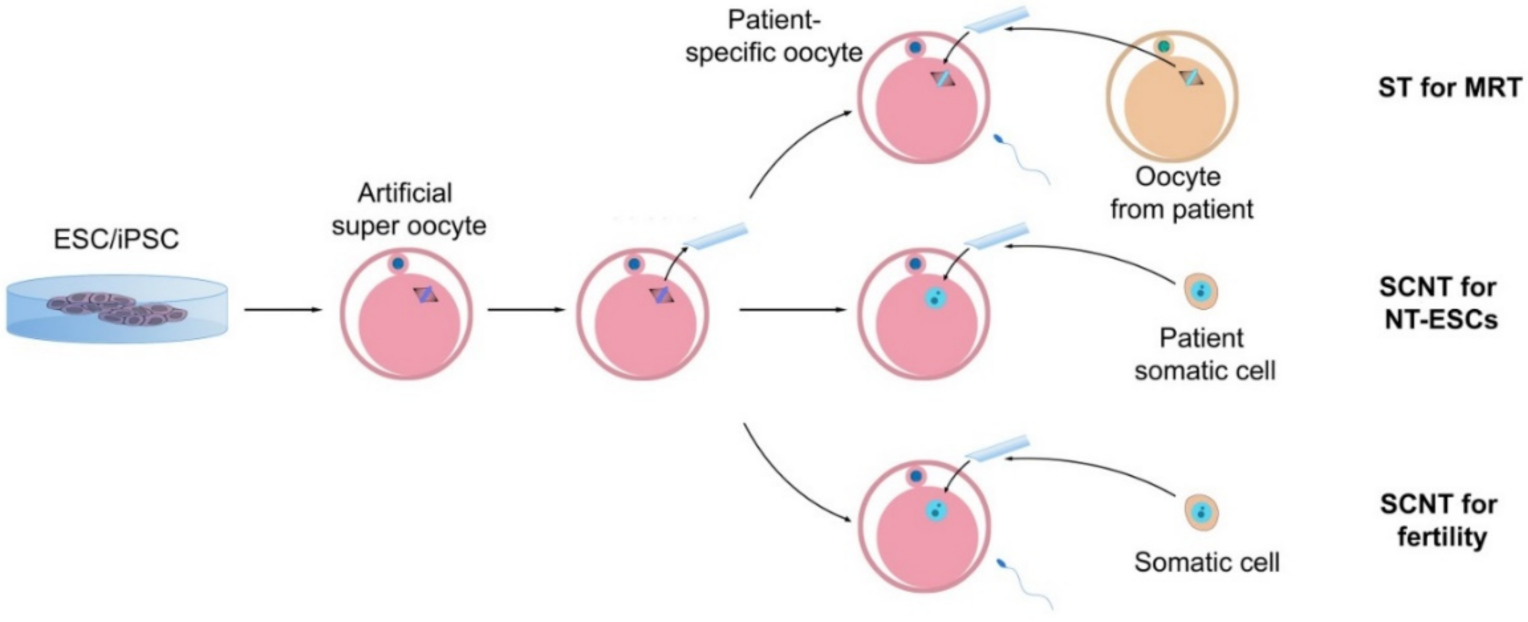
5. Challenges and Future Scenarios
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahamondes, L.; Makuch, M.Y. Infertility care and the introduction of new reproductive technologies in poor re-source settings. Reprod. Biol. Endocrinol. 2014, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Palmer, M.; Tanton, C.; Gibson, L.; Jones, K.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.; et al. Prevalence of infertility and help seeking among 15,000 women and men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, S.; Dancet, E.A.; van Pelt, A.M.; Hamer, G.; Repping, S. Artificial gametes: A systematic review of biologi-cal progress towards clinical application. Hum. Reprod. Update 2015, 21, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Chon, S.J.; Umair, Z.; Yoon, M.-S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- Lew, R.J.B.P.; Obstetrics, R.C. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 2–13. [Google Scholar] [CrossRef]
- França, M.M.; Mendonca, B.B. Genetics of primary ovarian insufficiency in the next-generation sequencing era. J. Endocr. Soc. 2020, 4, bvz037. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, D.; Brauner, R.; Lin, L.; De Perdigo, A.; Weryha, G.; Muresan, M.; Boudjenah, R.; Guerra-Junior, G.; Maciel-Guerra, A.T.; Achermann, J.; et al. Mutations inNR5A1Associated with Ovarian Insufficiency. N. Engl. J. Med. 2009, 360, 1200–1210. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, B.; Dong, Z.; Zhou, S.; Liu, Z.; Shi, G.; Cao, Y.; Xu, Y. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. Cell Death Dis. 2013, 4, e825. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.G.; Machado, A.Z.; Martins, C.N.; Domenice, S.; Costa, E.M.F.; Nishi, M.Y.; Ferraz-De-Souza, B.; Jorge, S.A.C.; Pereira, C.A.; Soardi, F.C.; et al. Homozygous Inactivating Mutation in NANOS3 in Two Sisters with Primary Ovarian Insufficiency. BioMed Res. Int. 2014, 2014, 787465–787468. [Google Scholar] [CrossRef] [Green Version]
- Rajkovic, A.; Pangas, S.A.; Ballow, D.; Suzumori, N.; Matzuk, M.M. NOBOX Deficiency Disrupts Early Folliculogenesis and Oocyte-Specific Gene Expression. Science 2004, 305, 1157–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Choi, Y.; Zhao, H.; Simpson, J.L.; Chen, Z.J.; Rajkovic, A. NOBOX homeobox mutation causes prema-ture ovarian failure. Am. J. Hum. Genet. 2007, 81, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Chen, Z.-J.; Qin, Y.; Shi, Y.; Wang, S.; Choi, Y.; Simpson, J.L.; Rajkovic, A. Transcription Factor FIGLA is Mutated in Patients with Premature Ovarian Failure. Am. J. Hum. Genet. 2008, 82, 1342–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patiño, L.C.; Walton, K.L.; Mueller, T.D.; Johnson, K.E.; Stocker, W.; Richani, D.; Agapiou, D.; Gilchrist, R.B.; Laissue, P.; Harrison, C.A. BMP15 Mutations Associated With Primary Ovarian Insufficiency Reduce Expression, Activity, or Synergy with GDF9. J. Clin. Endocrinol. Metab. 2017, 102. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 Is a Critical Specifier of Human Primordial Germ Cell Fate. Cell 2014, 160, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, M.; Snow, M.; McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar] [CrossRef]
- Saitou, M.; Yamaji, M. Germ cell specification in mice: Signaling, transcription regulation, and epigenetic consequences. Reproduction 2010, 139, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.; Andersen, C.B.; Richard, F.; Méhats, C.; Chun, S.-Y.; Horner, K.; Jin, C.; Tsafriri, A. Role of cyclic nucleotide signaling in oocyte maturation. Mol. Cell. Endocrinol. 2002, 187, 153–159. [Google Scholar] [CrossRef]
- Norris, R.P.; Ratzan, W.J.; Freudzon, M.; Mehlmann, L.M.; Krall, J.; Movsesian, M.A.; Wang, H.; Ke, H.; Nikolaev, V.; Jaffe, L.A. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009, 136, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa Cell Ligand NPPC and Its Receptor NPR2 Maintain Meiotic Arrest in Mouse Oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Dunn, N.R.; Hogan, B.L. Bone morphogenetic protein 4 in the extraembryonic mesoderm is re-quired for allantois development and the localization and survival of primordial germ cells in the mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 13739–13744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, M.; Barton, S.C.; Surani, A. A molecular programme for the specification of germ cell fate in mice. Nature 2002, 418, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, K.D.; Dunn, N.R.; Robertson, E.J. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tis-sues and germ cell formation. Development 2001, 128, 3609–3621. [Google Scholar] [CrossRef]
- Chu, G.C.; Dunn, N.R.; Anderson, D.C.; Oxburgh, L.; Robertson, E.J. Differential requirements for Smad4 in TGFβ-dependent patterning of the early mouse embryo. Development 2004, 131, 3501–3512. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Matzuk, M.M. Smad5 is required for mouse primordial germ cell development. Mech. Dev. 2001, 104, 61–67. [Google Scholar] [CrossRef]
- Yamaguchi, T.P.; Takada, S.; Yoshikawa, Y.; Wu, N.; McMahon, A.P. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999, 13, 3185–3190. [Google Scholar] [CrossRef] [Green Version]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.C.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef]
- Yamaji, M.; Seki, Y.; Kurimoto, K.; Yabuta, Y.; Yuasa, M.; Shigeta, M.; Yamanaka, K.; Ohinata, Y.; Saitou, M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat. Genet. 2008, 40, 1016–1022. [Google Scholar] [CrossRef]
- Weber, S.; Eckert, D.; Nettersheim, D.; Gillis, A.J.; Schäfer, S.; Kuckenberg, P.; Ehlermann, J.; Werling, U.; Biermann, K.; Looijenga, L.H.; et al. Critical Function of AP-2gamma/TCFAP2C in Mouse Embryonic Germ Cell Maintenance1. Biol. Reprod. 2010, 82, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, M.; Acampora, D.; Vojtek, M.; Yuan, D.; Simeone, A.; Chambers, I. OTX2 restricts entry to the mouse germline. Nature 2018, 562, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kehler, J.; Tolkunova, E.; Koschorz, B.; Pesce, M.; Gentile, L.; Boiani, M.; Lomelí, H.; Nagy, A.; McLaughlin, K.J.; Schöler, H.; et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runyan, C.; Schaible, K.; Molyneaux, K.; Wang, Z.; Levin, L.; Wylie, C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development 2006, 133, 4861–4869. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Tsuda, M.; Kiso, M.; Saga, Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev. Biol. 2008, 318, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.S.; Toyooka, Y.; Akasu, R.; Katoh-Fukui, Y.; Nakahara, Y.; Suzuki, R.; Yokoyama, M.; Noce, T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000, 14, 841–853. [Google Scholar] [CrossRef]
- Soyal, S.M.; Amleh, A.; Dean, J.J.D. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle for-mation. Development 2000, 127, 4645–4654. [Google Scholar] [CrossRef]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Liu, J.-G.; Hoja, M.-R.; Wilbertz, J.; Nordqvist, K.; Hӧӧg, C. Female Germ Cell Aneuploidy and Embryo Death in Mice Lacking the Meiosis-Specific Protein SCP3. Science 2002, 296, 1115–1118. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Litscher, E.S.J.G. Zona pellucida genes and proteins: Essential players in mammalian oogenesis and fer-tility. Genes 2021, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J.; Schroeder, A.C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and devel-opment to live young after growth, maturation, and fertilization in vitro. Biol. Reprod. 1989, 41, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eppig, J.J.; O’Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hirao, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A Revised Protocol for In Vitro Development of Mouse Oocytes from Primordial Follicles Dramatically Improves Their Developmental Competence1. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.Y.; Lei, L.; Shikanov, A.; Shea, L.D.; Woodruff, T.K. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertility 2010, 93, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Mochida, N.; Akatani-Hasegawa, A.; Saka, K.; Ogino, M.; Hosoda, Y.; Wada, R.; Sawai, H.; Shibahara, H. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013, 146, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Gougeon, A. Dynamics of follicular growth in the human: A model from preliminary results. Hum. Reprod. 1986, 1, 81–87. [Google Scholar] [CrossRef]
- Edwards, R.G. Maturation in vitro of Mouse, Sheep, Cow, Pig, Rhesus Monkey and Human Ovarian Oocytes. Nature 1965, 208, 349–351. [Google Scholar] [CrossRef]
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 1991, 55, 109–113. [Google Scholar] [CrossRef]
- Trounson, A.; Wood, C.; Kausche, A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil. Steril. 1994, 62, 353–362. [Google Scholar] [CrossRef]
- Barnes, F.L.; Crombie, A.; Gardner, D.K.; Kausche, A.; Lacham-Kaplan, O.; Suikkari, A.-M.; Tiglias, J.; Wood, C.; Trounson, A.O. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum. Reprod. 1995, 10, 3243–3247. [Google Scholar] [CrossRef]
- Hovatta, O.; Silye, R.; Abir, R.; Krausz, T.; Winston, R.M. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum. Reprod. 1997, 12, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Hovatta, O.; Wright, C.; Krausz, T.; Hardy, K.; Winston, R.M. Human primordial, primary and secondary ovarian fol-licles in long-term culture: Effect of partial isolation. Hum. Reprod. 1999, 14, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Barrett, S.L.; West-Farrell, E.; Kondapalli, L.A.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 2009, 24, 2531–2540. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports human oo-cyte meiotic maturation. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Banc, A.; Woodruff, T.K.; Shea, L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydro-gel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009, 103, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Paulino, L.R.; de Assis, E.I.; Azevedo, V.A.; Silva, B.R.; da Cunha, E.V.; Silva, J.R. Why Is It So Difficult To Have Com-petent Oocytes from In vitro Cultured Preantral Follicles? Reprod. Sci. 2022, 1–14. [Google Scholar] [CrossRef]
- Laronda, M.M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosden, R.G.; Laing, S.C.; Felicio, L.S.; Nelson, J.F.; Finch, C.E. Imminent Oocyte Exhaustion and Reduced Follicular Recruitment Mark the Transition to Acyclicity in Aging C57BL/6J Mice. Biol. Reprod. 1983, 28, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Faddy, M.J.M. Follicle dynamics during ovarian ageing. Mol. Cell. Endocrinol. 2000, 163, 43–48. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko-Tarui, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Skaznik-Wikiel, M.; Tilly, J.C.; Lee, H.J.; Niikura, Y.; Kaneko-Tarui, T.; Johnson, J.; Tilly, J.L. Serious doubts over “Eggs forever?”. Differentiation 2007, 75, 93–99. [Google Scholar] [CrossRef]
- White, Y.A.R.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wang, L.; Kang, J.X.; Xie, W.; Li, X.; Wu, C.; Xu, B.; Wu, J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol. Hum. Reprod. 2013, 20, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Gosden, R.G. Germline stem cells in the postnatal ovary: Is the ovary more like a testis? Hum. Reprod. Update 2004, 10, 193–195. [Google Scholar] [CrossRef] [Green Version]
- Greenfeld, D.A.; Klock, S.C. Disclosure decisions among known and anonymous oocyte donation recipients. Fertil. Steril. 2004, 81, 1565–1571. [Google Scholar] [CrossRef]
- Telfer, E.E.; Gosden, R.G.; Byskov, A.G.; Spears, N.; Albertini, D.; Andersen, C.Y.; Anderson, R.; Braw-Tal, R.; Clarke, H.; Gougeon, A.; et al. On Regenerating the Ovary and Generating Controversy. Cell 2005, 122, 821–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wu, J.J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. MHR Basic Sci. Reprod. Med. 2016, 22, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Satirapod, C.; Ohguchi, Y.; Park, E.-S.; Woods, D.C.; Tilly, J.L. Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Sci. Rep. 2017, 7, 10011. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, K.; Bhartiya, D.; Anand, S.; Bhutda, S. Mouse ovarian very small embryonic-like stem cells resist chemo-therapy and retain ability to initiate oocyte-specific differentiation. Reprod. Sci. 2015, 22, 884–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeilian, Y.; Atalay, A.; Erdemli, E. Putative germline and pluripotent stem cells in adult mouse ovary and their in vitro differentiation potential into oocyte-like and somatic cells. Zygote 2017, 25, 358–375. [Google Scholar] [CrossRef]
- Hubner, K.; Fuhrmann, G.; Christenson, L.K.; Kehler, J.; Reinbold, R.; De La Fuente, R.; Wood, J.; Strauss, J.F., III; Boiani, M.; Scholer, H.R. Derivation of oocytes from mouse embryonic stem cells. Sci. Adv. 2003, 300, 1251–1256. [Google Scholar]
- Lacham-Kaplan, O.; Chy, H.; Trounson, A. Testicular Cell Conditioned Medium Supports Differentiation of Embryonic Stem Cells into Ovarian Structures Containing Oocytes. Stem Cells 2005, 24, 266–273. [Google Scholar] [CrossRef]
- Qing, T.; Shi, Y.; Qin, H.; Ye, X.; Wei, W.; Liu, H.; Ding, M.; Deng, H. Induction of oocyte-like cells from mouse embry-onic stem cells by co-culture with ovarian granulosa cells. Differentiation 2007, 75, 902–911. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, P.; Cao, J.; Zhu, S.; Li, Y.; Zheng, L.; Chen, X.; Feng, L. Dazl Promotes Germ Cell Differentiation from Embryonic Stem Cells. J. Mol. Cell Biol. 2009, 1, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Suzuki, T.; Nagamatsu, G.; Yabukami, H.; Ikegaya, M.; Kishima, M.; Kita, H.; Imamura, T.; Nakashima, K.; Nishinakamura, R.; et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science 2021, 373. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from Oocytes Derived from in Vitro Primordial Germ Cell–like Cells in Mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaki, F.; Hayashi, K.; Ohta, H.; Kurimoto, K.; Yabuta, Y.; Saitou, M. Induction of mouse germ-cell fate by transcrip-tion factors in vitro. Nature 2013, 501, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Günesdogan, U.; Zylicz, J.; Tang, W.W.C.; Sengupta, R.; Kobayashi, T.; Kim, S.; Butler, R.; Dietmann, S.; Surani, M.A. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 2016, 529, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Miyauchi, H.; Ohta, H.; Nagaoka, S.; Nakaki, F.; Sasaki, K.; Hayashi, K.; Yabuta, Y.; Nakamura, T.; Yamamoto, T.; Saitou, M. Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J. 2017, 36, 3100–3119. [Google Scholar] [CrossRef]
- Tian, C.; Liu, L.; Ye, X.; Fu, H.; Sheng, X.; Wang, L.; Wang, H.; Heng, D.; Liu, L. Functional oocytes derived from granulosa cells. Cell Rep. 2019, 29, 4256–4267.e4259. [Google Scholar] [CrossRef] [Green Version]
- Hamazaki, N.; Kyogoku, H.; Araki, H.; Miura, F.; Horikawa, C.; Hamada, N.; Shimamoto, S.; Hikabe, O.; Nakashima, K.; Kitajima, T.S.; et al. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 2020, 589, 264–269. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Skutella, T.; Hren, M.; Gruden, K.; Cvjeticanin, B.; Vogler, A.; Šinkovec, J. Isolation of Small SSEA-4-Positive Putative Stem Cells from the Ovarian Surface Epithelium of Adult Human Ovaries by Two Different Methods. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Silvestris, E.; Cafforio, P.; D’Oronzo, S.; Felici, C.; Silvestris, F.; Loverro, G. In vitro differentiation of human oo-cyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Hum. Reprod. 2018, 33, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Virant-Klun, I.; Zech, N.; Rožman, P.; Vogler, A.; Cvjetičanin, B.; Klemenc, P.; Maličev, E.; Meden-Vrtovec, H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008, 76, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, Characterization, and Spontaneous Differentiation In Vitro of Very Small Embryonic-Like Putative Stem Cells in Adult Mammalian Ovary. Stem Cells Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I. Functional testing of primitive oocyte-like cells developed in ovarian surface epithelium cell culture from small VSEL-like stem cells: Can they be fertilized one day? Stem Cell Rev. 2018, 14, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.T.; Bodnar, M.S.; Fox, M.; Rodriquez, R.T.; Abeyta, M.J.; Firpo, M.T.; Pera, R.A.R. Spontaneous differentia-tion of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004, 13, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Aflatoonian, B.; Ruban, L.; Jones, M.; Aflatoonian, R.; Fazeli, A.; Moore, H.D. In vitro post-meiotic germ cell develop-ment from human embryonic stem cells. Hum. Reprod. 2009, 24, 3150–3159. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.; Xiong, J.; Ye, M.; Qin, X.; Li, L.; Cheng, S.; Luo, M.; Peng, J.; Dong, J.; Tang, F.; et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat. Commun. 2017, 8, 15680. [Google Scholar] [CrossRef]
- Irie, N.; Kim, S.; Surani, M.A. Human germline development from pluripotent stem cells in vitro. J. Mamm. Ova Res. 2016, 33, 79–87. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [Green Version]
- Sugawa, F.; Araúzo-Bravo, M.J.; Yoon, J.; Kim, K.P.; Aramaki, S.; Wu, G.; Stehling, M.; Psathaki, O.E.; Hübner, K.; Schöler, H.R. Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile. EMBO J. 2015, 34, 1009–1024. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.J.; Woods, D.C.; Tilly, J.L. Implications and current limitations of oogenesis from female germline or oogonial stem cellsinadult-mammalianovaries. Cells 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embry-onic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Parrott, J.A.; Kim, G.; Skinner, M.K. Expression and action of kit ligand/stem cell factor in normal human and bovine ovarian surface epithelium and ovarian cancer. Biol. Reprod. 2000, 62, 1600–1609. [Google Scholar] [CrossRef] [Green Version]
- Bukovsky, A.; Svetlikova, M.; Caudle, M.R. Oogenesis in cultures derived from adult human ovaries. Reprod. Biol. Endocrinol. 2005, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukovsky, A.; Caudle, M.R.; Svetlikova, M.; Upadhyaya, N.B. Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod. Biol. Endocrinol. 2004, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Telfer, E.E.; Albertini, D.F. The quest for human ovarian stem cells. Nat. Med. 2012, 18, 353–354. [Google Scholar] [CrossRef]
- Obata, Y.; Kono, T.J. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J. Biol. Chem. 2002, 277, 5285–5289. [Google Scholar] [CrossRef] [Green Version]
- Kurimoto, K.; Saitou, M. Germ cell reprogramming. Curr. Top. Dev. Biol. 2019, 135, 91–125. [Google Scholar]
- Ohta, H.; Kurimoto, K.; Okamoto, I.; Nakamura, T.; Yabuta, Y.; Miyauchi, H.; Yamamoto, T.; Okuno, Y.; Hagiwara, M.; Shirane, K.; et al. In vitro expansion of mouse primordial germ cell-like cells recapitulates an epigenetic blank slate. EMBO J. 2017, 36, 1888–1907. [Google Scholar] [CrossRef]
- Kurimoto, K.; Yabuta, Y.; Hayashi, K.; Ohta, H.; Kiyonari, H.; Mitani, T.; Moritoki, Y.; Kohri, K.; Kimura, H.; Yamamoto, T.; et al. Quantitative Dynamics of Chromatin Remodeling during Germ Cell Specification from Mouse Embryonic Stem Cells. Cell Stem Cell 2015, 16, 517–532. [Google Scholar] [CrossRef] [Green Version]
- Matoba, S.; Ogura, A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol. Reprod. 2011, 84, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaren, A. Primordial germ cells in the mouse. Dev. Biol. 2003, 262, 1–15. [Google Scholar] [CrossRef] [Green Version]
- van Pelt, A.M.; de Rooij, D.G. Synchronization of the seminiferous epithelium after vitamin A replacement in vita-min A-deficient mice. Biol. Reprod. 1990, 43, 363–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livera, G.; Rouiller-Fabre, V.; Valla, J.; Habert, R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol. Cell. Endocrinol. 2000, 165, S0303–S7207. [Google Scholar] [CrossRef]
- Kee, K.; Gonsalves, J.M.; Clark, A.T.; Pera, R.A. Development. Bone morphogenetic proteins induce germ cell differ-entiation from human embryonic stem cells. Stem Cells Dev. 2006, 15, 831–837. [Google Scholar] [CrossRef]
- West, F.D.; Machacek, D.W.; Boyd, N.L.; Pandiyan, K.; Robbins, K.R.; Stice, S.L. Enrichment and differentiation of hu-man germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells 2008, 26, 2768–2776. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, N.; Qing, T.; Cheng, J.; Bai, Z.; Shi, Y.; Ding, M.; Deng, H. Live Offspring Produced by Mouse Oocytes Derived from Premeiotic Fetal Germ Cells1. Biol. Reprod. 2006, 75, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Li, L.; Bai, Z.; Pan, Q.; Ding, M.; Deng, H. In vitro development of mouse fetal germ cells into mature oocytes. Reproduction 2007, 134, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Toyooka, Y.; Tsunekawa, N.; Akasu, R.; Noce, T. Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. USA 2003, 100, 11457–11462. [Google Scholar] [CrossRef] [Green Version]
- Geijsen, N.; Horoschak, M.; Kim, K.; Gribnau, J.; Eggan, K.; Daley, G.Q. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 2003, 427, 148–154. [Google Scholar] [CrossRef]
- Magnúsdóttir, E.; Dietmann, S.; Murakami, K.; Günesdogan, U.; Tang, F.; Bao, S.; Diamanti, E.; Lao, K.; Gottgens, B.; Surani, M.A. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat. Cell Biol. 2013, 15, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anchan, R.; Gerami-Naini, B.; Lindsey, J.S.; Ho, J.W.; Kiezun, A.; Lipskind, S.; Ng, N.; LiCausi, J.A.; Kim, C.S.; Brezina, P.J. Efficient differentiation of steroidogenic and germ-like cells from epigenetically-related iPSCs derived from ovari-an granulosa cells. PLoS ONE 2015, 10, e0119275. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Lerou, P.H.; Zhao, R.; Huo, H.; Daley, G.Q. Generation of human-induced pluripotent stem cells. Nat. Pro-Toc. 2008, 3, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Galic, Z.; Conway, A.E.; Lindgren, A.; Van Handel, B.J.; Magnusson, M.; Richter, L.; Teitell, M.A.; Mikkola, H.K.; Lowry, W.E. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is signif-icantly improved by coculture with human fetal gonadal cells. Stem Cells 2009, 27, 783–795. [Google Scholar] [CrossRef] [Green Version]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Reijo Pera, R.A. Human DAZL, DAZ and BOULE genes modulate pri-mordial germ-cell and haploid gamete formation. Nature 2009, 462, 222–225. [Google Scholar] [CrossRef]
- Panula, S.; Medrano, J.V.; Kee, K.; Bergström, R.; Nguyen, H.N.; Byers, B.; Wilson, K.D.; Wu, J.C.; Simon, C.; Hovatta, O.J.; et al. Human germ cell differentiation from fetal-and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 752–762. [Google Scholar] [CrossRef]
- Medrano, J.V.; Ramathal, C.; Nguyen, H.N.; Simon, C.; Pera, R.A.R. Divergent RNA-binding Proteins, DAZL and VASA, Induce Meiotic Progression in Human Germ Cells Derived in Vitro. Stem Cells 2011, 30, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Wongtrakoongate, P.; Jones, M.; Gokhale, P.J.; Andrews, P.W. STELLA facilitates differentiation of germ cell and en-dodermal lineages of human embryonic stem cells. PLoS ONE 2013, 8, e56893. [Google Scholar] [CrossRef] [Green Version]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yokobayashi, S.; Kojima, Y.; Saitou, M.J. Generation of human oogonia from induced plu-ripotent stem cells in culture. Nat. Protoc. 2020, 15, 1560–1583. [Google Scholar] [CrossRef]
- Stringer, J.M.; Western, P.S. A step toward making human oocytes. Nat. Biotechnol. 2019, 37, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.P.; Chang, C.-C. Artificial gametes. Theriogenology 2007, 67, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Matsuda, J.; Yanagimachi, R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc. Natl. Acad. Sci. USA 1994, 91, 7460–7462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic Sperm Injection in the Mouse1. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Kubelka, M.; Moor, R.M. The behaviour of mitotic nuclei after transplantation to early meiotic ooplasts or mitotic cyto-plasts. Zygote 1997, 5, 219–227. [Google Scholar] [CrossRef]
- Wakayama, T.; Hayashi, Y.; Ogura, A. Participation of the female pronucleus derived from the second polar body in full embryonic development of mice. J. Reprod. Fertil. 1997, 110, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Fulka Jr, J.; Tesařík, J.; Loi, P.; Moor, R.M. Manipulating the human embryo: Cell cycle checkpoint controls. Cloning 2000, 2, 1–7. [Google Scholar] [CrossRef]
- Tesarik, J.; Nagy, Z.; Sousa, M.; Mendoza, C.; Abdelmassih, R. Fertilizable oocytes reconstructed from patient’s so-matic cell nuclei and donor ooplasts. Reprod. BioMedicine Online 2001, 2, 160–164. [Google Scholar] [CrossRef]
- Fulka Jr, J.; Loi, P.; Fulka, H.; Kren, R.; Dean, W.; Mrazek, M.; Reik, W.J.C.; Cells, S. Nucleus replacement in mammalian oocytes. Cloning Stem Cells 2002, 4, 181–187. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Oliveira, S.A.; Abdelmassih, V.; Abdelmassih, R. Novel use of laser to assist ICSI for patients with fragile oocytes: A case report. Reprod. Biomed. Online 2002, 4, 27–31. [Google Scholar] [CrossRef]
- Palermo, G.D.; Takeuchi, T.; Rosenwaks, Z. Oocyte-induced haploidization. Reprod. Biomed. Online 2002, 4, 237–242. [Google Scholar] [CrossRef]
- Chang, C.-C.; Nagy, Z.P.; Abdelmassih, R.; Yang, X.; Tian, X.C. Nuclear and microtubule dynamics of G2/M somatic nuclei during haploidization in germinal vesicle-stage mouse oocytes. Biol. Reprod. 2004, 70, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Galat, V.; Ozen, S.; Rechitsky, S.; Kuliev, A.; Verlinsky, Y. Cytogenetic analysis of human somatic cell haploidization. Reprod. Biomed. Online 2005, 10, 199–204. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Chang, C.-C. Current advances in artificial gametes. Reprod. Biomed. Online 2005, 11, 332–339. [Google Scholar] [CrossRef]
- Lee, Y.; Trout, A.; Marti-Gutierrez, N.; Kang, S.; Xie, P.; Mikhalchenko, A.; Kim, B.; Choi, J.; So, S.; Han, J. Haploidy in so-matic cells is induced by mature oocytes in mice. Commun. Biol. 2022, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Geens, M.; Spits, C. Genetic and epigenetic instability in human pluripotent stem cells. Hum. Reprod. Updat. 2012, 19, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Bar, S.; Benvenisty, N. Epigenetic aberrations in human pluripotent stem cells. EMBO J. 2019, 38, e101033. [Google Scholar] [CrossRef]
- Keller, A.; Dziedzicka, D.; Zambelli, F.; Markouli, C.; Sermon, K.; Spits, C.; Geens, M. Genetic and epigenetic factors which modulate differentiation propensity in human pluripotent stem cells. Hum. Reprod. Updat. 2018, 24, 162–175. [Google Scholar] [CrossRef]
- Tapia, N.; Schöler, H.R. Molecular obstacles to clinical translation of iPSCs. Cell Stem Cell 2016, 19, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Wang, X.; Tippner-Hedges, R.; Ma, H.; Folmes, C.; Gutierrez, N.M.; Lee, Y.; Van Dyken, C.; Ahmed, R.; Li, Y.; et al. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell Stem Cell 2016, 18, 625–636. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C.; Mendoza-Tesarik, R. Fertility. Human artificial oocytes from patients’ somatic cells: Past, pre-sent and future. Reproduction 2021, 2, H1–H8. [Google Scholar]
- Andrews, P.W.; Ben-David, U.; Benvenisty, N.; Coffey, P.; Eggan, K.; Knowles, B.B.; Nagy, A.; Pera, M.; Reubinoff, B.; Rugg-Gunn, P.J.; et al. Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. Stem Cell Rep. 2017, 9, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popp, B.; Krumbiegel, M.; Grosch, J.; Sommer, A.; Uebe, S.; Kohl, Z.; Plötz, S.; Farrell, M.; Trautmann, U.; Kraus, C.; et al. Need for high-resolution Genetic Analysis in iPSC: Results and Lessons from the ForIPS Consortium. Sci. Rep. 2018, 8, 17201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.-Q.; Vaidya, A.; Sun, W.; Maeda, A.; Kern, T.; Palczewski, K.; Lu, Z.-R. Non-viral gene therapy for Stargardt disease with ECO/pRHO-ABCA4 self-assembled nanoparticles. Mol. Ther. 2020, 28, 293–303. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Antao, A.M.; Karapurkar, J.K.; Lee, D.R.; Kim, K.-S.; Ramakrishna, S. Disease modeling and stem cell im-munoengineering in regenerative medicine using CRISPR/Cas9 systems. Comput. Struct. Biotechnol. J. 2020, 18, 3649–3665. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Grünewald, J.; Szalay, R.; Shih, J.; Anzalone, A.V.; Lam, K.C.; Shen, M.W.; Petri, K.; Liu, D.R.; Joung, J.K. PrimeDesign software for rapid and simplified design of prime editing guide RNAs. Nat. Commun. 2021, 12, 1034. [Google Scholar] [CrossRef]
- Wang, S.; Min, Z.; Ji, Q.; Geng, L.; Su, Y.; Liu, Z.; Hu, H.; Wang, L.; Zhang, W.; Suzuiki, K.J.P.; et al. Rescue of premature ag-ing defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction. Protein 2020, 11, 1–22. [Google Scholar]
- Riesenberg, S.; Chintalapati, M.; Macak, D.; Kanis, P.; Maricic, T.; Pääbo, S. Simultaneous precise editing of multiple genes in human cells. Nucleic Acids Res. 2019, 47, e116. [Google Scholar] [CrossRef]
- Seki, T.; Fukuda, K. Methods of induced pluripotent stem cells for clinical application. World J. Stem Cells 2015, 7, 116. [Google Scholar] [CrossRef]
- Reader, K.L.; Stanton, J.-A.L.; Juengel, J.L. The Role of Oocyte Organelles in Determining Developmental Competence. Biology 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, E. Stem cells and reproduction. BMB Rep. 2019, 52, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Christodoulaki, A.; Boel, A.; Tang, M.; De Roo, C.; Stoop, D.; Heindryckx, B. Prospects of Germline Nuclear Transfer in Women With Diminished Ovarian Reserve. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Tachibana, M.; Sparman, M.; Sritanaudomchai, H.; Ma, H.; Clepper, L.; Woodward, J.; Li, Y.; Ramsey, C.; Kolotushkina, O.; Mitalipov, S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 2009, 461, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craven, L.; Tuppen, H.A.; Greggains, G.D.; Harbottle, S.J.; Murphy, J.L.; Cree, L.M.; Murdoch, A.P.; Chinnery, P.F.; Taylor, R.W.; Lightowlers, R.N.; et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 2010, 465, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.-S.; Sritanaudomchai, H.; et al. Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paull, D.; Emmanuele, V.; Weiss, K.A.; Treff, N.; Stewart, L.; Hua, H.; Zimmer, M.; Kahler, D.J.; Goland, R.S.; Noggle, S.A.; et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 2012, 493, 632–637. [Google Scholar] [CrossRef]
- Kang, E.; Wu, J.; Gutierrez, N.M.; Koski, A.; Tippner-Hedges, R.; Agaronyan, K.; Platero-Luengo, A.; Martinez-Redondo, P.; Ma, H.; Lee, Y.; et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 2016, 540, 270–275. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef] [Green Version]


| Mouse | |||
|---|---|---|---|
| Source | Outcome | Markers for Selection | References |
| OSC | MII oocyte | Scp3, Spo11, Dmc1 | [68] |
| MII oocyte | Oct4, Blimp1, Dazl, Stella, Mvh, Fragilis, Rex1 | [44] | |
| FGSCs | Ddx4, Dazl, Blimp1, Stella, Fragilis | [72] | |
| Oocyte | Oct4, Ddx4, Stella, Nobox, Sohlh1, Zp3 | [73] | |
| VSELC | OLC | Mvh, Stra8, Msy-2 | [74] |
| OLC | Ddx4, Dazl, and Zp3 | [75] | |
| mESC | OLC | Zp2, Zp3, Figα | [76] |
| OLC | Zp3, Scp3, Figα | [77] | |
| OLC | Zp3, Gdf9, Figα | [78] | |
| OLC | Zp1, Zp2, Zp3, Gdf9 | [79] | |
| MII oocyte | Prdm1, Stella, Ddx4, Scp3 | [80] | |
| miPSC | PGCLC | Prdm1, Stella | [81] |
| GV oocyte | Prdm1, Prdm14 | [82] | |
| PGCLC | Prdm1, Prdm14, Tfap2c, Nanos3, Stella | [83] | |
| PGCLC | Prdm1, Nanos3 | [84] | |
| MII oocyte | Stella, Dazl | [85] | |
| Oocyte | Scp3, Stra8, Nobox | [86] | |
| MII oocyte | Prdm1, Dazl, Vasa | [87] | |
| MII oocyte | Gdf9 | [88] | |
| Human | |||
| OSE | OLC | ZP, CK18 | [51] |
| OLC | SSEA-4 | [89] | |
| OSC | OLC | GDF-9, SYCP3 | [90] |
| Oocyte | DDX4, KIT, YBX2, NOBOX, LHX8, GDF9, ZP1 ZP2, ZP3 | [67] | |
| VSELC | OLC | OCT4A/B, VASA, ZP | [91] |
| OLC | c-KIT, DAZL, GDF-9, VASA, ZP4 | [92] | |
| OLC | OCT4A, ZP3 | [93] | |
| hESC | OLC | VASA, BOL, SCP1, SCP3, GDF9, TEKT1 | [94] |
| OLC | VASA, SSEA-1, DAZL | [95] | |
| Primary oocyte | OCT4, NANOG, PRDM14, VASA | [96] | |
| hiPSC | PGCLC | PRDM1, STELLA | [97] |
| PGCLC | PRDM1, PRDM14, TFAP2C, T, SOX17, SOX15 | [98] | |
| PGCLC | PRDM1, STELLA, KLF2, TFAP2C | [99] | |
| Oogonium | DAZL, DDX4, REC8, SYCP3, STRA8 | [100] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oqani, R.K.; So, S.; Lee, Y.; Ko, J.J.; Kang, E. Artificial Oocyte: Development and Potential Application. Cells 2022, 11, 1135. https://doi.org/10.3390/cells11071135
Oqani RK, So S, Lee Y, Ko JJ, Kang E. Artificial Oocyte: Development and Potential Application. Cells. 2022; 11(7):1135. https://doi.org/10.3390/cells11071135
Chicago/Turabian StyleOqani, Reza K., Seongjun So, Yeonmi Lee, Jung Jae Ko, and Eunju Kang. 2022. "Artificial Oocyte: Development and Potential Application" Cells 11, no. 7: 1135. https://doi.org/10.3390/cells11071135









