Morphological and Structural Control of Organic Monolayer Colloidal Crystal Based on Plasma Etching and Its Application in Fabrication of Ordered Gold Nanostructured Arrays
Abstract
:1. Introduction
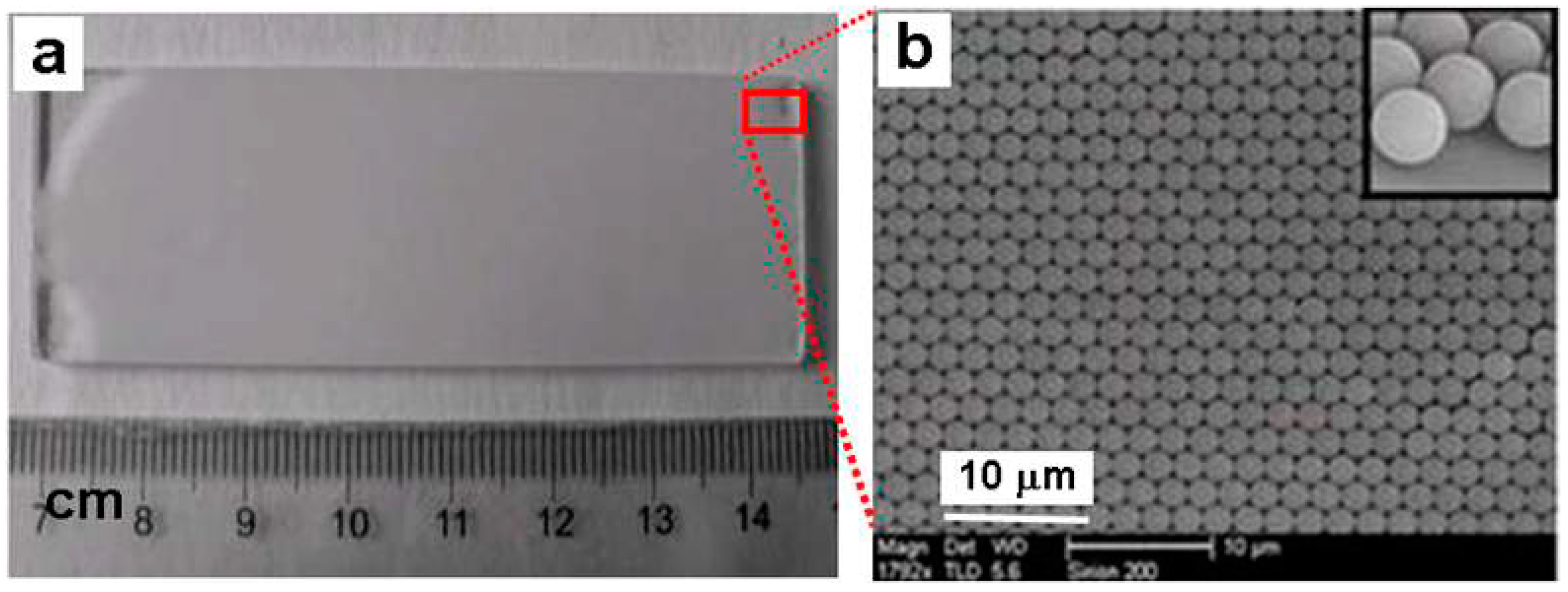
2. Close-Packed Polystyrene (PS) Monolayer Colloidal Crystal and Its Transferrable Property
3. Structural Manipulation Based on Plasma Etching
3.1. Etching of the As-Prepared Close-Packed Colloidal Monolayer
3.2. Etching of the Heated Close-Packed Colloidal Monolayer
4. Fabrication of Gold Nanostructured Arrays
4.1. Triangular Nanoplate Arrays from the Close-Packed Monolayer Colloidal Template
4.2. Opening-Nanoshell Arrays from Non-Close-Packed Monolayer Colloidal Crystals
4.3. Au Binary-Structure Arrays from the Colloidal Template with Dualistic Structure
5. Summary and Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reichenbach, P.; Eng, L.M.; Georgi, U.; Voit, B. 3D-steering and superfocusing of second-harmonic radiation through plasmonic nano antenna arrays. J. Laser. Appl. 2012, 24, 042005. [Google Scholar] [CrossRef]
- Vontobel, P.O.; Robinett, W.; Kuekes, P.J.; Stewart, D.R.; Straznicky, J.; Williams, R.S. Writing to and reading from a nano-scale crossbar memory based on memristors. Nanotechnology 2009, 20, 425204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Song, J.H.; Liu, J.; Wang, Z.L. Direct current nanogenerator driven by ultrasonic wave. Science 2007, 316, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Tian, M.W.; Nysten, B.; Jonas, A.M. Regular arrays of highly ordered ferroelectric polymer nanostructures for non-volatile low-voltage memories. Nature Mater. 2009, 8, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.J.; Heo, S.H.; Li, C.C.; Choi, Y.K.; Cai, W.P.; Cho, S.O. Superhydrophobic bionic surfaces with hierarchical microsphere/SWCNT composite arrays. Langmuir 2007, 23, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.-F.; Murray-Méthot, M.-P.; Live, L.S. Nanohole arrays in chemical analysis: Manufacturing methods and applications. Analyst 2010, 135, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Debrina, J.; Abhijit, M.; Goutam, D. High Raman enhancing shape-tunable Ag nanoplates in alumina: A reliable and efficient SERS technique. ACS Appl. Mater. Inter. 2012, 4, 3330–3334. [Google Scholar]
- Liu, G.Q.; Cai, W.P.; Kong, L.C.; Duan, G.T.; Li, Y.; Wang, J.J.; Zuo, G.M.; Cheng, Z.X. Standing Ag nanoplate-built hollow microphere arrays controllable structural paramerters and strong SERS performances. J. Mater. Chem. 2012, 22, 3177–3184. [Google Scholar] [CrossRef]
- Fu, R.R.; Liu, G.Q.; Jia, C.; Li, X.H.; Tang, X.; Duan, G.T.; Li, Y.; Cai, W.P. Fabrication of silver nanoplate built hierarchical turreted ordered array and the application in trace analyses. Chem. Comm. 2015, 51, 6609–6612. [Google Scholar]
- Xu, S.H.; Fei, G.T.; Zhu, X.G.; Wang, B.A.; Wu, B.; Zhang, L.D. A facile and universal way to fabricate superlattice nanowire arrays. Nanotechnology 2011, 22, 265602. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.B.; Lei, Y. Sub-100-nm nanoparticle arrays with perfect ordering and tunable and uniform dimensions fabricated by combining nanoimprinting with ultrathin alumina membrane technique. ACS Nano 2014, 8, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Gu, Y.N.; Wang, L.; Ge, H.X.; Wu, W.; Xia, Q.F.; Yuan, C.S.; Chen, Y.; Cui, B.; Williams, R.S. Hybrid nanoimprint−soft lithography with sub-15 nm resolution. Nano Lett. 2009, 9, 2306–2310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F. Nanofabrication by electron beam lithography and its applications: A review. Microelectro Eng. 2015, 135, 57–72. [Google Scholar] [CrossRef]
- Li, Y.; Duan, G.T.; Liu, G.Q.; Cai, W.P. Physical processes-aided periodic micro/ nanostructured arrays by colloidal template technique: fabrication and application. Chem. Soc. Rev. 2013, 42, 3614–3627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Duan, G.T.; Li, Y.; Liu, G.Q.; Cai, W.P. Wet etching-assisted colloidal lithography: A general strategy toward nanodisk and nanohole arrays on arbitrary substrates. ACS Appl. Mater. Inter. 2014, 6, 9207–9213. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Cai, W.P.; Kong, L.C.; Duan, G.T.; Li, Y.; Wang, J.J.; Cheng, Z.X. Trace detection of cyanide based on SERS effect of Ag nanoplate-built hollow microsphere arrays. J. Hazard. Mater. 2013, 248, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, W.P.; Duan, G.T. Ordered micro/nanostructured arrays based on the monolayer colloidal crystals. Chem. Mater. 2008, 20, 615–624. [Google Scholar] [CrossRef]
- Ye, X.; Qi, L. Two-dimensionally patterned nanostructures based on monolayer colloidal crystals: Controllable fabrication, assembly, and applications. Nano Today 2011, 6, 608–631. [Google Scholar] [CrossRef]
- Vogel, N.; Weiss, C.K.; Landfester, K. From soft to hard: the generation of functional and complex colloidal monolayers for nanolithography. Soft Matter. 2012, 8, 4044–4061. [Google Scholar] [CrossRef]
- Vogel, N.; Retsch, M.; Fustin, C.-A.; Campo, A.; Jonas, U. Advances in Colloidal Assembly: The Design of Structure and Hierarchy in Two and Three Dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.Q.; Cai, W.P.; Li, Y.; Cao, B.Q.; Lei, Y.; Zhang, L.D. Morphology-controlled growth of large-area two-dimensional ordered pore arrays. Adv. Funct. Mater. 2004, 14, 283–288. [Google Scholar] [CrossRef]
- Liu, Z.F.; Jin, Z.G.; Liu, W.; Liu, X.X. Ordered porous ZnO thin films formed by dip-coating method using PS templates. J. Sol-Gel. Sci. Tech. 2006, 40, 25–30. [Google Scholar] [CrossRef]
- Dai, Z.F.; Li, Y.; Duan, G.T.; Jia, L.C.; Cai, W.P. Phase diagram, design of monolayer binary colloidal crystals, and their fabrication based on ethanol-assisted self-assembly at the air/water interface. ACS Nano 2012, 6, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, Q.; Shen, D. Co-self-assembly of binary colloidal crystals at the air-water interface. ACS Appl. Mater. Interface 2010, 2, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Cai, W.P.; Kong, L.C.; Duan, G.T.; Lü, F.J. Vertically cross-linking silver nanoplate arrays with controllable density based on seed-assisted electrochemical growth and their structurally enhanced SERS activity. J. Mater. Chem. 2010, 20, 767–772. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Li, X.; Zhang, J.H.; Li, W.; Zhang, X.M.; Yang, B. Tunable two-dimensional non-close-packed microwell arrays using colloidal crystals as templates. Langmuir 2007, 23, 8272–8276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; McFarland, M.J. Wafer-scale periodic nanohole arrays templated from two-dimensional nonclose-packed colloidal crystals. J. Am. Chem. Soc. 2005, 127, 3710–3711. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Duan, G.T.; Cai, W.P. Controllable fabrication and tunable magnetism of nickel nanostructured ordered porous arrays. J. Phys. Chem. C 2009, 113, 3973–3977. [Google Scholar] [CrossRef]
- Liu, G.Q.; Li, X.H.; Wang, W.B.; Zhou, F.; Duan, G.T.; Li, Y.; Xu, Z.K.; Cai, W.P. Gold binary-structured arrays based on monolayer colloidal crystal and their optical properties. Small 2014, 10, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Sinitskii, A.; Tour, J.M. Patterning graphene through the self-assembled templates: toward periodic two-dimensional graphene nanostructures with semiconductor properties. J. Am. Chem. Soc. 2010, 132, 14730–14732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sasaki, T.; Shimizu, Y.; Koshizaki, N. Hexagonal-close-packed, hierarchical amorphous TiO2 nanocolumn arrays: Transferability, enhanced photocatalytic activity, and superamphiphilicity without UV irradiation. J Am. Chem. Soc. 2008, 130, 14755–14762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Hicks, E.M.; Zhao, J.; Schatz, G.C.; Duyne, R.P.V. Electrochemical tuning of silver nanoparticles fabricated by nanosphere lithography. Nano Lett. 2005, 5, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Murray, W.A.; Suckling, J.R.; Barnes, W.L. Overlayers on silver nanotriangles: Field confinement and spectral position of localized surface plasmon resonances. Nano Lett. 2006, 6, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Li, Y.; Duan, G.T.; Wang, J.J.; Cai, W.P. Tunable surface plasmon resonance and strong SERS performances of Au opening nanoshell ordered arrays. ACS Appl. Mater. Interface 2012, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Feldmann, C.; Wanner, M.; Gerthsen, D. Nanoscale gold hollow spheres through a microemulsion approach. Small 2007, 3, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, J.L.; Lu, W.; Wang, X.P.; Wang, B.; Zhang, S.Y.; Hou, J.G. Necklace-like noble-metal hollow nanoparticle chains: synthesis and tunable optical properties. Adv. Mater. 2007, 19, 2172–2176. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yang, B. Patterning colloidal crytals and nanostructure arrays by soft lithography. Adv. Funct. Mater. 2010, 20, 3411–3424. [Google Scholar] [CrossRef]
- Konorov, S.O.; Addison, C.J.; Schulze, H.G.; Turner, R.F.B.; Blades, M.W. Hollow-core photonic crystal fiber-optic probes for Raman spectroscopy. Opt. Lett. 2006, 31, 1911–1913. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: New York, NY, USA, 1995. [Google Scholar]
- Haynes, C.L.; Duyne, R.P.V. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Sun, F.Q.; Cai, W.P.; Li, Y.; Duan, G.T.; Nichols, W.T.; Liang, C.H.; Koshizaki, N.; Fang, Q.; Boyd, I.W. Laser morphological manipulation of gold nanoparticle arrays formed by nanosphere lithography. Appl. Phys. B 2005, 81, 765–768. [Google Scholar] [CrossRef]
- Duan, G.T.; Lv, F.J.; Cai, W.P.; Luo, Y.Y.; Li, Y.; Liu, G.Q. General synthesis of 2D ordered hollow sphere arrays based on nonshadow deposition dominated colloidal lithography. Langmuir 2010, 26, 6295–6302. [Google Scholar] [CrossRef] [PubMed]
- Draine, B.T.; Flatau, P.J. Discrete-dipole approximation for scattering calculation. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]










© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Cai, W. Morphological and Structural Control of Organic Monolayer Colloidal Crystal Based on Plasma Etching and Its Application in Fabrication of Ordered Gold Nanostructured Arrays. Crystals 2016, 6, 126. https://doi.org/10.3390/cryst6100126
Liu G, Cai W. Morphological and Structural Control of Organic Monolayer Colloidal Crystal Based on Plasma Etching and Its Application in Fabrication of Ordered Gold Nanostructured Arrays. Crystals. 2016; 6(10):126. https://doi.org/10.3390/cryst6100126
Chicago/Turabian StyleLiu, Guangqiang, and Weiping Cai. 2016. "Morphological and Structural Control of Organic Monolayer Colloidal Crystal Based on Plasma Etching and Its Application in Fabrication of Ordered Gold Nanostructured Arrays" Crystals 6, no. 10: 126. https://doi.org/10.3390/cryst6100126
APA StyleLiu, G., & Cai, W. (2016). Morphological and Structural Control of Organic Monolayer Colloidal Crystal Based on Plasma Etching and Its Application in Fabrication of Ordered Gold Nanostructured Arrays. Crystals, 6(10), 126. https://doi.org/10.3390/cryst6100126





