A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting
Abstract
:1. Introduction
1.1. Bioprinting Strategies
1.2. Bioinks
3D Printing Mechanism
1.3. Representative Bioprinting Techniques
2. Hydrogels in Bioprinting
2.1. Hydrogel Crosslinking Techniques
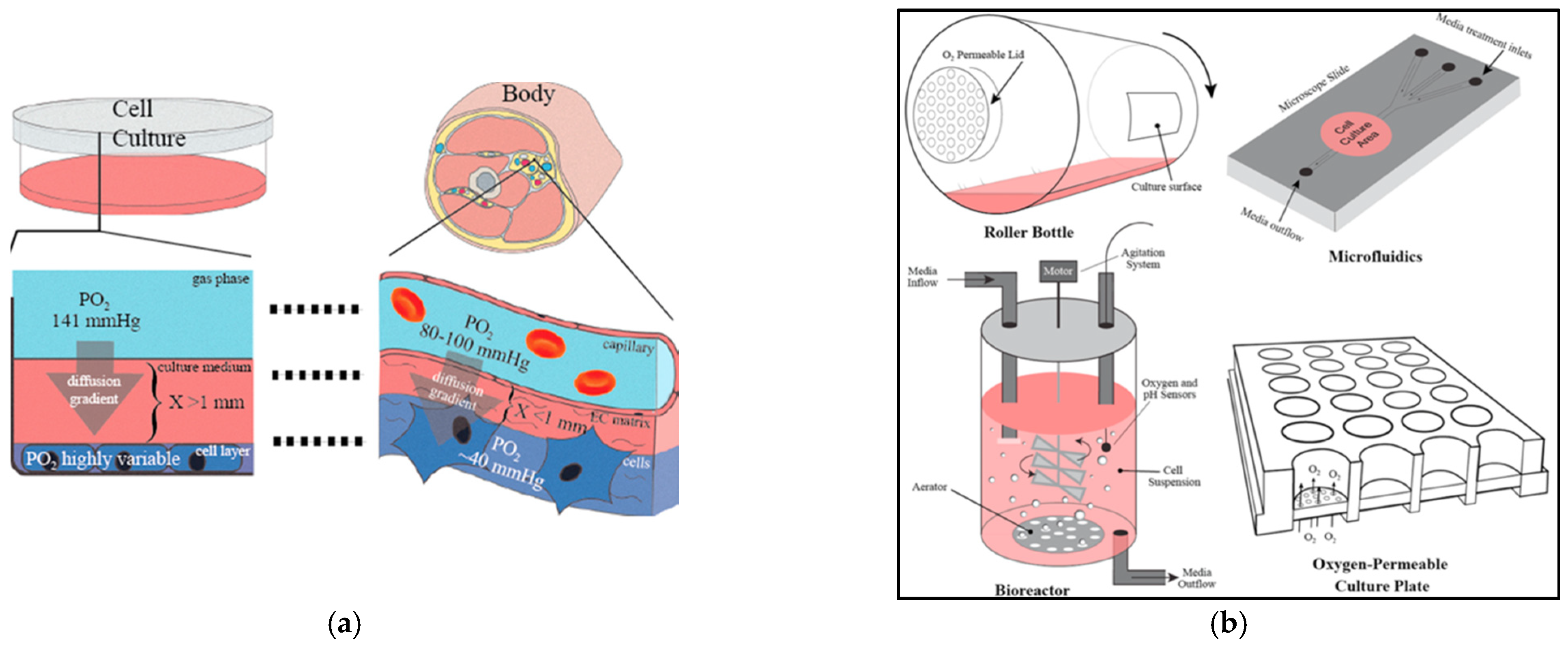
2.2. Nutrient Transport in Hydrogels
- J—flux.
- D—diffusion coefficient of solute (nutrient and/or oxygen) in the medium.
- c—concentration of the solute (nutrient and/or oxygen) in the medium.
- x—distance.
2.3. Hydrogel Swelling Kinetics
2.4. Biological Properties of Hydrogels
2.4.1. Biocompatibility
2.4.2. Biodegradation
2.5. Rheological Properties
2.5.1. Viscosity
- δP—difference between the applied pressure on the bioink and the ambient pressure.
- L—length of the nozzle tip.
- Q—flow rate or scan speed.
- μ—viscosity of the bioink.
- r—radius of the nozzle tip.
2.5.2. Shear-Thinning
3. Hydrogel-Based Bioinks
3.1. Classification of Scaffold-Based Bioinks
3.1.1. Natural Bioinks
Type 1 Collagen
- Collagen Bioprinted 3D Heart Model:
Fibrin
- Role of Fibrin in Neural Cell Printing:
Hyaluronic Acid (HA)
- Role of Hyaluronic Acid in Osteogenic Induction:
Alginate
- 3D-Printed Bionic Ear Using Alginate:
Agarose
- 3D Model of an Arterial Bifurcation Trunk Using Agarose:
Dextran
Chitosan
Cellulose
Silk Fibroin (SF)
- 3D Tracheal Cartilaginous Ring Fabrication Using Silk Fibroin:
Gelatin
- Bioprosthetic Ovarian Constructs Using Gelatin:
GelMA
- GelMA-Based Nerve Guidance Conduits for Peripheral Nerve Injury:
Matrigel
3.1.2. Synthetic Bioinks
Polyethylene Glycol (PEG)
- Alveolar Model Using PEG:
Poloxamers
Poly Vinyl Alcohol (PVA)
Silicone
3.1.3. Composite Bioinks
Collagen–Gold Nanowires in Muscle-Tissue Engineering
Agarose–Laponite Nanosilicates in Fibroblast Culturing
Alginate–Poloxamer Composites for Cartilage Formation
PVA–Chitosan for the Fabrication of Bioprinted Cornea
Silk–Gelatin Hybrids for Bioprinting Skin
Matrigel–Agarose Composites in Biomimetic Intestinal Model
Cellulose–Carbon Nanotubes for In Vitro Neural Growth Models
GelMA–Chitosan–Dextran for Osteogenesis and Wound Healing
4. Prospective and Outlook
4.1. Biomaterial Limitation
4.2. Lack of Printing Resolution

4.3. Multi-Component Printing
4.4. Vascularization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mironov, V. Printing Technology to Produce Living Tissue. Expert Opin. Biol. Ther. 2003, 3, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and Organ Printing 2: Fusion of Cell Aggregates in Three-Dimensional Gels. Anat. Rec. 2003, 272, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.; Mostafa, S.; Pakvasa, M.; Luu, H.; Lee, M.; Wolf, J.; Ameer, G.; He, T.; Reid, R. 3D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Gene Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Poldervaart, M.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.; Öner, F.; Dhert, W.; Vermonden, T.; Alblas, J. 3D Bioprinting of Methacrylated Hyaluronic Acid (MeHA) Hydrogel with Intrinsic Osteogenicity. Public Libr. Sci. One 2017, 12, e0177628. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Ma, X.; Bernaerts, K.; Ren, P.; Hu, W.; Zhang, T. Expansion of Ovarian Cancer Stem-like Cells in Poly(ethylene glycol)-Cross-Linked Poly(methyl vinyl ether-alt-maleic acid) and Alginate Double-Network Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 3310–3326. [Google Scholar] [CrossRef]
- Lawlor, K.; Vanslambrouck, J.; Higgins, J.; Chambon, A.; Bishard, K.; Arndt, D.; Er, P.; Wilson, S.; Howden, S.; Tan, K.; et al. Cellular Extrusion Bioprinting Improves Kidney Organoid Reproducibility and Conformation. Nat. Mater. 2020, 20, 260–271. [Google Scholar] [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.; Shim, J.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, 1905505. [Google Scholar] [CrossRef]
- Mannoor, M.; Jiang, Z.; James, T.; Kong, Y.; Malatesta, K.; Soboyejo, W.; Verma, N.; Gracias, D.; McAlpine, M. 3D Printed Bionic Ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 2009, 26, 163–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laschke, M.W.; Menger, M.D. Vascularization in Tissue Engineering: Angiogenesis Versus Inosculation. Eur. Surg. Res. 2012, 48, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Burdick, J.; Cho, D.; Derby, B.; Gelinsky, M.; Heilshorn, S.; Jüngst, T.; Malda, J.; Mironov, V.; Nakayama, K.; et al. A Definition of Bioinks and their Distinction from Biomaterial Inks. Biofabrication 2019, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Anseth, K. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2008, 26, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merceron, T.K.; Murphy, S.V. Chapter 14—Hydrogels for 3D Bioprinting Applications. In Essentials of 3D Biofabrication and Translation; Elsevier/Academic Press: Cambridge, MA, USA, 2015; pp. 249–270. ISBN 9780128009727. [Google Scholar]
- Lai, K.; Renneberg, R.; Mak, W. High Efficiency Single-Step Biomaterial-Based Microparticle Fabrication via Template-Directed Supramolecular Coordination Chemistry. Green Chem. 2016, 18, 1715–1723. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, C.; Chu, P.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Diggle, B.; Tan, M.; Viktorova, J.; Bennett, C.; Connal, L. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.D. An Overview of Laser-Assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Ramesh, S.; Harrysson, O.; Rao, P.; Tamayol, A.; Cormier, D.; Zhang, Y.; Rivero, I. Extrusion Bioprinting: Recent Progress, Challenges, and Future Opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Hinton, T.; Jallerat, Q.; Palchesko, R.; Park, J.; Grodzicki, M.; Shue, H.; Ramadan, M.; Hudson, A.; Feinberg, A. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tan, Y.; Kiran, R.; Tor, S.; Zhou, K. Submerged and Non-Submerged 3D Bioprinting Approaches for the Fabrication of Complex Structures with the Hydrogel Pair GelMA and Alginate/Methylcellulose. Addit. Manuf. 2021, 37, 101640. [Google Scholar] [CrossRef]
- McCormack, A.; Highley, C.; Leslie, N.; Melchels, F. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaeser, A.; Duarte Campos, D.; Weber, M.; Neuss, S.; Theek, B.; Fischer, H.; Jahnen-Dechent, W. Biofabrication Under Fluorocarbon: A Novel Freeform Fabrication Technique to Generate High Aspect Ratio Tissue-Engineered Constructs. BioRes. Open Access 2013, 2, 374–384. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.; Urbanska, A.; Kaplan, D.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.A.; Otoni, C.G.; Espitia, P.J.P. Chapter 3—Fundamentals of Chitosan-Based Hydrogels: Elaboration and Characterization Techniques. In Materials for Biomedical Engineering: Hydrogels and Polymer-Based Scaffolds, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–81. ISBN 9780128169025. [Google Scholar]
- Hoffman, A. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.; Feijen, J.; van Blitterswijk, C.; Dijkstra, P.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T. Cross-Linked Polymer Synthesis. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–11. ISBN 978-3-642-29648-2. [Google Scholar]
- Gasperini, L.; Mano, J.; Reis, R. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Int. 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennink, W.; van Nostrum, C. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Vinchhi, P.; Rawal, S.; Patel, M. Chapter 19—Biodegradable Hydrogels. In Drug Delivery Devices and Therapeutic Systems; Academic Press: Cambridge, MA, USA, 2021; pp. 395–419. ISBN 9780128198384. [Google Scholar]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Lin, C.; Metters, A. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Figueiredo, L.; Le Visage, C.; Weiss, P.; Yang, J. Quantifying Oxygen Levels in 3D Bioprinted Cell-Laden Thick Constructs with Perfusable Microchannel Networks. Polymers 2020, 12, 1260. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K. Perfluorocarbon-based oxygen carriers: From Physics to Physiology. Pflügers Arch. Eur. J. Physiol. 2020, 473, 139–150. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.; Camci-Unal, G. Breathing Life into Engineered Tissues using Oxygen-Releasing Biomaterials. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.C.; Aleman, J.; Mutkus, L.; Skardal, A. A Mechanically Robust Thixotropic Collagen and Hyaluronic Acid Bioink Supplemented with Gelatin Nanoparticles. Bioprinting 2019, 16, E00058. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.; Inci, I.; Zhang, Y.; Khademhosseini, A.; Dokmeci, M. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L. Correlating Rheological Properties and Printability of Collagen Bioinks: The Effects of Riboflavin Photocrosslinking And pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef] [PubMed]
- De Melo, B.; Jodat, Y.; Cruz, E.; Benincasa, J.; Shin, S.; Porcionatto, M. Strategies to use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Barker, T.H. Fibrin-Based Biomaterials: Modulation of Macroscopic Properties through Rational Design at the Molecular Level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and Physical and Biological Properties of Fibrin Gel Derived from Human Plasma. Biomed. Mater. 2008, 3, 015001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Lee, J.; Yim, H.; Kim, G.; Chun, W. Development of Cell-laden 3D Scaffolds for Efficient Engineered Skin Substitutes by Collagen Gelation. R. Soc. Chem. Adv. 2016, 6, 21439–21447. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.; Mason, B.; Reinhart-King, C.; Bonassar, L. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Jang, K.; Hahn, S.; Park, J.; Jung, H.; Oh, K.; Park, K.; Yeom, J.; Park, S.; Kim, S.; et al. Three-Dimensional Bioprinting of Multilayered Constructs Containing Human Mesenchymal Stromal Cells for Osteochondral Tissue Regeneration in the Rabbit Knee Joint. Biofabrication 2016, 8, 014102. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of Collagen Type I-Hyaluronan Hybrid Bioink for 3D Bioprinted Liver Microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.; Fischer, K.; Polio, S.; Park, J.; Yoo, S. Three-Dimensional Bioprinting of Rat Embryonic Neural Cells. NeuroReport 2009, 20, 798–803. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Cubo, N.; Garcia, M.; del Cañizo, J.; Velasco, D.; Jorcano, J. 3D Bioprinting of Functional Human Skin: Production and in-vivo Analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.; Kießling, F.; Fischer, H. Engineering Biofunctional in-vitro Vessel Models Using a Multilayer Bioprinting Technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lee, S.; Cheng, H.; Yoo, J.; Atala, A. 3D Bioprinted Functional and Contractile Cardiac Tissue Constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Maloney, E.; Clark, C.; Sivakumar, H.; Yoo, K.; Aleman, J.; Rajan, S.; Forsythe, S.; Mazzocchi, A.; Laxton, A.; Tatter, S.; et al. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines 2020, 11, 208. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; He, Y.; Fu, J.; Liu, A.; Ma, L. Coaxial Nozzle-Assisted 3D Bioprinting with built-in Microchannels for Nutrients Delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Wüst, S.; Godla, M.; Müller, R.; Hofmann, S. Tunable Hydrogel Composite with Two-Step Processing in Combination with Innovative Hardware Upgrade for Cell-Based Three-Dimensional Bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef]
- He, Y.; Derakhshanfar, S.; Zhong, W.; Li, B.; Lu, F.; Xing, M.; Li, X. Characterization and Application of Carboxymethyl Chitosan-Based Bioink in Cartilage Tissue Engineering. J. Nanomater. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Wu, J.; Moochhala, S.M.; Tan, M.H.; Lu, J. Development of a Chitosan-Based Wound Dressing with Improved Hemostatic and Antimicrobial Properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, T.; Lee, O.J.; Lee, J.S.; Yoon, S.; et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.; Rutz, A.; Xiao, S.; Whelan, K.; Duncan, F.; Roth, E.; Woodruff, T.; Shah, R. A Bioprosthetic Ovary Created using 3D Printed Microporous Scaffolds Restores Ovarian Function in Sterilized Mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; Zhang, F.; et al. 3D Printing of Gelatin Methacrylate-Based Nerve Guidance Conduits with Multiple Channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A Three-Dimensional in-vitro Ovarian Cancer Coculture Model using a High-Throughput Cell Patterning Platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.; Corbett, D.; Sazer, D.; Fortin, C.; Zaita, A.; Greenfield, P.; Calafat, N.; Gounley, J.; Ta, A.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic Hydrogels as Bioinks for 3D Bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Luis, E.; Pan, H.; Bastola, A.; Bajpai, R.; Sing, S.; Song, J.; Yeong, W. 3D Printed Silicone Meniscus Implants: Influence of the 3D Printing Process on Properties of Silicone Implants. Polymers 2020, 12, 2136. [Google Scholar] [CrossRef]
- Roberts, J.J.; Martens, P.J. Chapter 9—Engineering Biosynthetic Cell Encapsulation Systems. In Biosynthetic Polymers for Medical Applications; Elsevier/Woodhead Publishing: Cambridge UK, 2016; pp. 205–239. ISBN 9781782421054. [Google Scholar]
- Cummings, C.L.; Gawlitta, D.; Nerem, R.M.; Stegemann, J.P. Properties of Engineered Vascular Constructs made from Collagen, Fibrin, and Collagen-Fibrin Mixtures. Biomaterials 2004, 25, 3699–3706. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.; Harrington, D.; Farach-Carson, M.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Cowman, M.; Schmidt, T.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef] [Green Version]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Mooney, D. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, F.; Kelly, D. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shamkhani, A.; Duncan, R. Radioiodination of Alginate via Covalently-Bound Tyrosinamide Allows Monitoring of its Fate In Vivo. J. Bioact. Compat. Polym. 1995, 10, 4–13. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hyu, H. Development and Evaluation of Polyvinyl Alcohol-Hydrogels as an Artificial Atrticular Cartilage for Orthopedic Implants. Materials 2010, 3, 2753–2771. [Google Scholar] [CrossRef] [Green Version]
- Cambria, E.; Brunner, S.; Heusser, S.; Fisch, P.; Hitzl, W.; Ferguson, S.; Wuertz-Kozak, K. Cell-Laden Agarose-Collagen Composite Hydrogels for Mechanotransduction Studies. Front. Bioeng. Biotechnol. 2020, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Nadernezhad, A.; Caliskan, O.; Topuz, F.; Afghah, F.; Erman, B.; Koc, B. Nanocomposite Bioinks Based on Agarose and 2D Nanosilicates with Tunable Flow Properties and Bioactivity for 3D Bioprinting. ACS Appl. Bio Mater. 2019, 2, 796–806. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J. Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [Green Version]
- Benwood, C.; Chrenek, J.; Kirsch, R.; Masri, N.; Richards, H.; Teetzen, K.; Willerth, S. Natural Biomaterials and their use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran Hydrogel Scaffolds Enhance Angiogenic Responses and Promote Complete Skin Regeneration During Burn Wound Healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [Green Version]
- Obi, S.; Masuda, H.; Akimaru, H.; Shizuno, T.; Yamamoto, K.; Ando, J.; Asahara, T. Dextran Induces Differentiation of Circulating Endothelial Progenitor Cells. Physiol. Rep. 2014, 2, e00261. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Wu, Q.; Therriault, D.; Heuzey, M. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Badhe, R.V.; Nipate, S.S. Chapter 5—Cellulosic Materials as Bioinks for 3D Printing Applications. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; Elsevier/Woodhouse Publishing: Duxford, UK, 2020; pp. 109–137. ISBN 9780128184714. [Google Scholar]
- Zainal, S.; Mohd, N.; Suhaili, N.; Anuar, F.; Lazim, A.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Charlesby, A. The Degradation of Cellulose by Ionizing Radiation. J. Polym. Sci. 1955, 15, 263–270. [Google Scholar] [CrossRef]
- Fontana, J.; De Souza, A.; Fontana, C.; Torriani, I.; Moreschi, J.; Gallotti, B.; De Souza, S.; Narcisco, G.; Bichara, J.; Farah, L. Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Kundu, S.; Dash, B.; Dash, R.; Kaplan, D. Natural Protective Glue Protein, Sericin Bioengineered by Silkworms: Potential for Biomedical and Biotechnological Applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Rodriguez, M.; Brown, J.; Giordano, J.; Lin, S.; Omenetto, F.; Kaplan, D. Silk Based Bioinks for Soft Tissue Reconstruction Using 3-Dimensional (3D) Printing with In-Vitro and In-Vivo Assessments. Biomaterials 2017, 117, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Galateanu, B.; Hudita, A.; Zaharia, C.; Bunea, M.-C.; Vasile, H.; Guga, M.-R.; Costache, M. Chapter 60—Silk-Based Hydrogels for Biomedical Applications. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 1791–1870. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Floren, M.; Migliaresi, C.; Motta, A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J. Funct. Biomater. 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kluge, J.; Leisk, G.; Kaplan, D. Sonication-induced Gelation of Silk Fibroin for Cell Encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.; Kim, I.; Zhang, K. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.U.; Sharmeen, S.; Biswas, S.; Ahmed, T.; Mallik, A.K.; Shahruzzaman, M.; Rahman, M.M. Chapter 54—Gelatin-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composite: A Reference Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 1601–1641. ISBN 978-3-319-77830-3. [Google Scholar]
- Liu, W.; Heinrich, M.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.; Yue, K.; Levett, P.; Klein, T.; Melchels, F.; Khademhosseini, A.; Hutmacher, D. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacryloyl–Based Hydrogels as Modular Tissue culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, H.; Martin, G. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Ma, S.; Wang, S.; Li, Q.; Leng, Y.; Wang, L.; Hu, G.-H. A Novel Method for Preparing Poly (Vinyl Alcohol) Hydrogels: Preparation, Characterization, and Application. Ind. Eng. Chem. Res. 2017, 56, 7971–7976. [Google Scholar] [CrossRef]
- Ma, R.; Xiong, D. Synthesis and Properties of Physically Crosslinked Poly (Vinyl Alcohol) Hydrogels. J. China Univ. Min. Technol. 2008, 18, 271–274. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.; Venkatraman, P.; Ramakrishna, S. Silicone-Based Biomaterials for Biomedical Applications: Antimicrobial Strategies and 3D Printing Technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Work, W.; Horie, K.; Hess, M.; Stepto, R. Definition of Terms Related to Polymer Blends, Composites, and Multiphase Polymeric Materials. Pure Appl. Chem. 2004, 76, 1985–2007. [Google Scholar] [CrossRef]
- Kim, W.; Jang, C.; Kim, G. A Myoblast-Laden Collagen Bioink with Fully Aligned Au Nanowires for Muscle-Tissue Regeneration. Nano Lett. 2019, 19, 8612–8620. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Arai, K.; Nakayama, K. Scaffold-Free Bio-3D Printing Using Spheroids as “Bio-Inks” for Tissue (Re-)Construction and Drug Response Tests. Adv. Healthc. Mater. 2020, 9, 1901831. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Burke, M.; Carter, B.; Davis, S.; Perriman, A. 3D Bioprinting Using a Templated Porous Bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Ulag, S.; Ilhan, E.; Sahin, A.; Karademir Yilmaz, B.; Kalaskar, D.; Ekren, N.; Kilic, O.; Nuzhet Oktar, F.; Gunduz, O. 3D Printed Artificial Cornea for Corneal Stromal Transplantation. Eur. Polym. J. 2020, 133, 109744. [Google Scholar] [CrossRef]
- Admane, P.; Gupta, A.; Jois, P.; Roy, S.; Chandrasekharan Lakshmanan, C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D Bioprinted Full-Thickness Skin Constructs Recapitulate Regulatory Signaling Pathways and Physiology of Human Skin. Bioprinting 2019, 15, e00051. [Google Scholar] [CrossRef]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-printing Cell-Laden Matrigel–Agarose Constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-Made Conductive Inks from Cellulose Nanofibrils for 3D Printing of Neural Guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Turner, P.; Murray, E.; McAdam, C.; McConnell, M.; Cabral, J. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.; Zhang, H.; Yin, R.; Yang, S.; Wei, J.; Zhang, W. Tyrosinase-Doped Bioink for 3D bioprinting of Living Skin Constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wu, Y.; Yu, M.; Aazmi, A.; Gao, L.; Xue, Q.; Luo, Y.; Zhou, H.; Zhang, B.; et al. 3D Bioprinted Hyaluronic Acid-Based Cell-Laden Scaffold for Brain Microenvironment Simulation. Bio-Des. Manuf. 2020, 3, 164–174. [Google Scholar] [CrossRef]
- Sakai, S.; Yoshii, A.; Sakurai, S.; Horii, K.; Nagasuna, O. Silk Fibroin Nanofibers: A Promising Ink Additive for Extrusion Three-Dimensional Bioprinting. Mater. Today Biol. 2020, 8, 100078. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, C.; Meyers, K.; Johnson, E.; Rajachar, R. Application of Composite Hydrogels to Control Physical Properties in Tissue Engineering and Regenerative Medicine. Gels 2018, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Karak, N. Chapter 1—Fundamentals of Polymers. In Vegetable Oil-Based Polymers: Properties, Processing and Applications, 1st ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 1–30. ISBN 9780857097149. [Google Scholar]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E. Advances in Interpenetrating Polymer Network Hydrogels and Their Applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Inamdar, A.; Cherukattu, J.; Anand, A.; Kandasubramanian, B. Thermoplastic-Toughened High-Temperature Cyanate Esters and their Application in Advanced Composites. Ind. Eng. Chem. Res. 2018, 57, 4479–4504. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Engineering of Tough Double Network Hydrogels. Macromol. Chem. Phys. 2016, 217, 1022–2036. [Google Scholar] [CrossRef]
- Kolesky, D.; Truby, R.; Gladman, A.; Busbee, T.; Homan, K.; Lewis, J. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Smith, L.; Li, P.; Holland, M.; Ekser, B. FABRICA: A Bioreactor Platform for Printing, Perfusing, Observing, & Stimulating 3D Tissues. Sci. Rep. 2018, 8, 7561. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.; Cho, D. Computer-Aided Multiple-Head 3D Printing System for Printing of Heterogeneous Organ/Tissue Constructs. Sci. Rep. 2016, 6, 21685. [Google Scholar] [CrossRef]
- Feng, F.; He, J.; Li, J.; Mao, M.; Li, D. Multicomponent Bioprinting of Heterogeneous Hydrogel Constructs Based on Microfluidic Printheads. Int. J. Bioprint. 2019, 5, 39. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2015, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Godsey, M.; Bhatia, S. Perfluorocarbons Enhance Oxygen Transport in Alginate-Based Hydrogels. Polym. Adv. Technol. 2014, 25, 1242–1246. [Google Scholar] [CrossRef]
- Brennan, M.; Rexius-Hall, M.; Eddington, D. A 3D-Printed Oxygen Control Insert for a 24-Well Plate. Public Libr. Sci. One 2015, 10, e0137631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gey, G.O. An Improved Technic for Massive Tissue Culture. Am. J. Cancer Res. 1993, 17, 752–756. [Google Scholar] [CrossRef]
- Erdem, A.; Darabi, M.; Nasiri, R.; Sangabathuni, S.; Ertas, Y.; Alem, H.; Hosseini, V.; Shamloo, A.; Nasr, A.; Ahadian, S.; et al. 3D Bioprinting: 3D Bioprinting of Oxygenated Cell-Laden Gelatin Methacryloyl Constructs (Adv. Healthcare Mater. 15/2020). Adv. Healthc. Mater. 2020, 9, 2070047. [Google Scholar] [CrossRef]
- Place, L.T.; Domann, E.F.; Case, J.A. Limitations of Oxygen Delivery in Cell Cultures: An Underappreciated Problem in Basic and Translational Research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef] [PubMed]
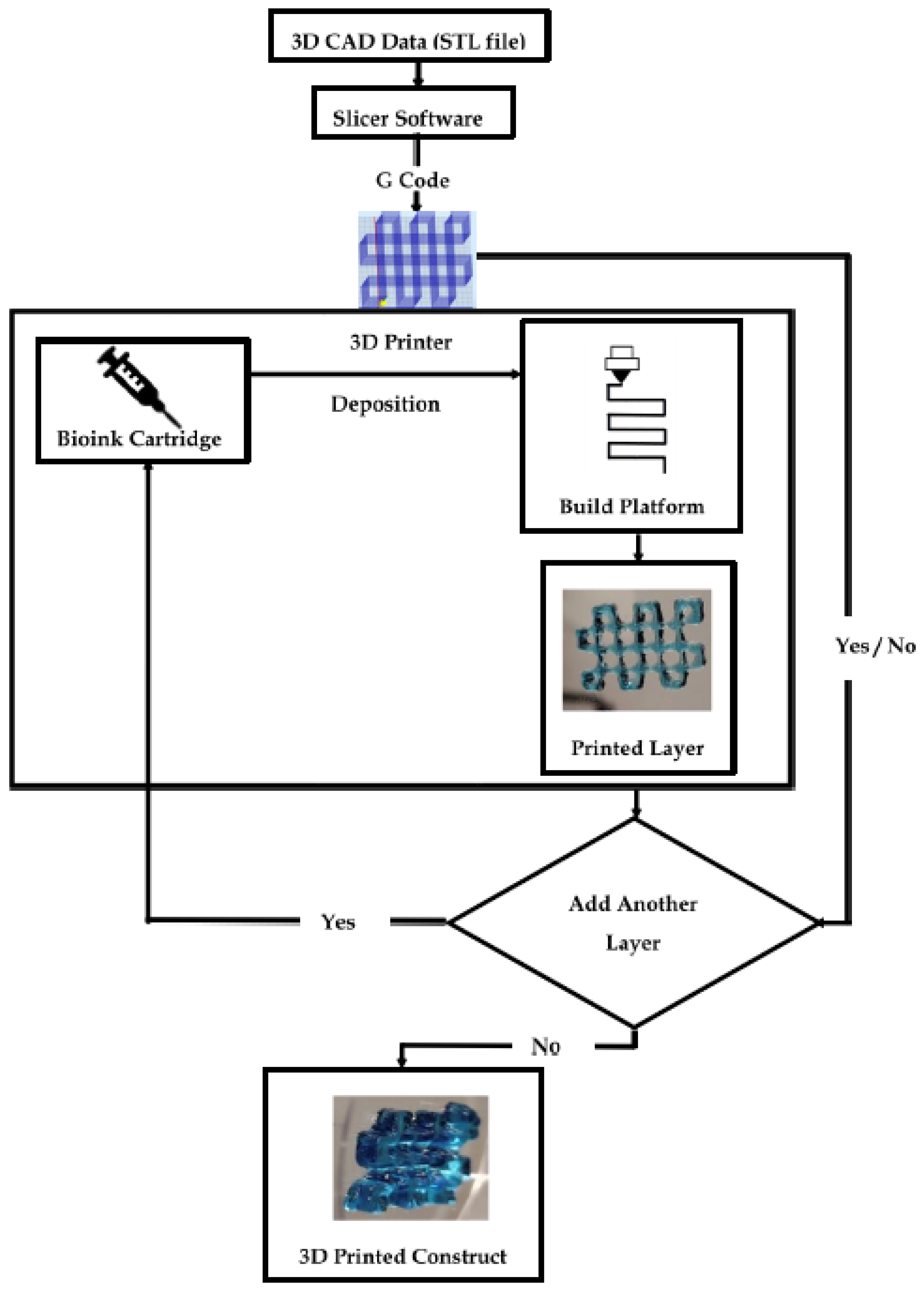



















| Categories | Laser-Assisted Bioprinting (LAB) | Stereolithography (ST) | Inkjet Printing | Extrusion Printing |
|---|---|---|---|---|
| Energy Source | Laser beam in the UV wavelength range [14]. | Light (visible and UV) [3]. | Thermal, electrostatic, electromagnetic, and piezoelectric forces [14]. | Pneumatic or mechanical pressure [14]. |
| Working Mechanism | Nozzle-free printing technique that uses laser beams to direct the bioink deposition [3]. | Nozzle-free procedure that uses UV or visible light [3,22]. | A non-contact printing process where droplets of biomaterial are injected in the presence of an appropriate energy source [14]. | Applied pressure produces continuous flow of bioink from the print nozzle [10]. Extrusion may occur through a heated nozzle (fused filament fabrication) or using external pressure (direct ink writing) [23]. |
| Requirements | Rapid gelation, high viscosity (1–300 mPa/s) [19]. | Addition of non-toxic, water- soluble photoinitiators and light absorbers to initiate photopolymerization [3]. | Material must be non-fibrous in its un-crosslinked state, with a low viscosity (3.5 to 12 mPa/s) [19]. | High viscosity (from 30 mPa/s to 60 × 10 7 mPa/s) [19]. |
| Common Biomaterials Used | Collagen, gelatin, fibrin, alginate [24]. | Curable acrylics, epoxies [3,22]. | Agarose, Matrigel [16]. | Hyaluronic acid [5,16], chitosan silk [20], polyethylene glycol, poloxamers [16]. |
| Print Resolution | 10–50 μm. One cell per droplet [14]. | 5–300 μm [14]. | 50–500 μm [18]. | 200–1000 μm [25] |
| Cell Viability Rate | >95% [3]. | >90% [3]. | >85% [3]. | As low as 40% [3]. |
| Advantage | No clogging issues due to nozzle-free, non-contact [3,14], heterogenous cell positioning ability with high accuracy [14]. | 1. Clog-free, with high accuracy [3,14]. 2. Enables printing of large-scale 3D models [21]. | Well suited for biomaterials with low viscosity, low cost, high printing speeds [14,19]. | Good structural integrity, allows for cell printing with high densities (>108 cells/mL) [25] and versatility [14]. |
| Disadvantage | Expensive, time-consuming, low stability [3,14,24]. | 1. Limited due to the lack of biocompatible and biodegradable light-sensitive polymers, and the cytotoxicity of photoinitiators [3]. 2. Slow printing rate [21]. | Frequent nozzle blockage [3]. | Distortion in cell morphology may occur due to the high pressure needed to extrude the viscous bioink; does not allow for spatial cell positioning [3,14]. |
| Categories | Physical Crosslinking | Chemical Crosslinking | Enzymatic Crosslinking |
|---|---|---|---|
| General Description | Reversible physical entanglements between polymer chains. | Robust bonding between polymers formed by the addition of external chemical agents. | Biological-derived natural catalytic factors that enable crosslinking in physiological conditions. |
| Crosslinkers Used | Temperature, pH, inherent molecular interactions (hydrogen, hydrophobic, and ionic bonding). | Photoinitiators (LAP, eosin Y, Irgacure), chemicals (genipin, glutaraldehyde). | Tyrosinase, transglutaminase, lysyl oxidase. |
| Advantages | Reversible, non-toxic. | Extremely stable, allows for control of mechanical strength. | Crosslinking is always carried under physiological conditions, and the majority of the enzymes used are common to those that catalyze in vivo reactions, are non-toxic, and can be used to crosslink opaque materials. Enzymes do not require light to be activated. |
| Disadvantages | Unstable and easily disrupted with changes in temperature, pH, or ionic concentration. | Crosslinkers used may induce cellular toxicity or may require additional components to be activated (e.g., Irgacure is only activated by UV light). | Crosslinking is not tunable. |
| Biomaterials Used | Alginate, agarose, collagen, Matrigel. | Chitosan, gelatin methacrylate, hyaluronic acid, silk. | Fibrin, gelatin, elastin, PEG. |
| Polymer | Gelation Mechanism | Printing Method | Printing Concentration (w/v) | Cell Viability Rate (%) | Application | Ref. |
|---|---|---|---|---|---|---|
| Natural | ||||||
| Collagen Type I | Self-assembly neutralization in acid medium + thermal gelation (37 °C), photopolymerization, chemical modification | Inkjet, extrusion | 35 mg/mL | High | Cardiovascular tissue [4], Skin [49], cartilage [50], bone [51], liver [52], nerve regeneration model [53], cornea [54]. | 4 |
| Fibrin | Enzymatic thrombin + CaCl2 + and genipin | Inkjet, extrusion | 10 to 60 mg/mL | 75% | Neural constructs [55], skin [56], blood vessels [57], cardiac tissues [58]. | 55 |
| Hyaluronic Acid | Photopolymerization, click chemistry, chemical group functionalization (thiol, methacrylate etc.), crosslinking agents (gold, PEG) | Extrusion | 2.5% | 64.4 ± 12.2% (21 days) | Bone and cartilage engineering [5], tumor models [59]. | 5 |
| Alginate | Divalent ions | Inkjet, extrusion, laser | 1.5 to 3% | High (90 to 95%) (10 weeks) | 3D-printed ear [9], vascular tissue [60], and bone printing [61]. | 9 |
| Agarose | Thermal crosslinking (31 to 36 °C) | Inkjet, extrusion | 0.3% | 97% | Arterial bifurcation [29] | 29 |
| Chitosan | Chemical crosslinking Schiff-base reaction (genepin, glutaraldehyde) photo crosslinking | Extrusion | 90:10 (ratio of chitosan to EDTA- modified chitosan) | 95.6 ± 1.3% (36 h) | Cartilage engineering [62], drug delivery [63], wound repair [64]. | 62 |
| Silk | Physical crosslinking (hydrophobic, hydrophilic, and hydrogen-bonding interactions), photo polymerization, enzymatic (horseradish peroxidase) | Extrusion | 30% (chemically modified by methacrylate) | High (4 weeks) | Tracheal ring [65]. | 65 |
| Gelatin | Thermal gelation (4 °C), chemical crosslinking by Schiff-base reaction (glutaraldehyde), photopolymerization | Extrusion | 10% | 78.57 ± 3.57% (Day 8) | Bioprinted ovaries [66]. | 66 |
| Gelatin Methacrylate | Photo crosslinking, ionic interactions | Digital light printing, extrusion | 13.3% | High (Day 10) | Nerve guidance conduit [67]. | 67 |
| Matrigel | Thermal gelation (37 °C) | Inkjet, laser | 2% | 100% (72 h) | Co-cultures of ovarian tumor and human fibroblast cells [68]. | 68 |
| Synthetic | ||||||
| PEG | Physical, chemical, photo crosslinking | Extrusion | 20% | High | Alveolar model [69]. | 69 |
| Poloxamers (Pluronic (F-127)) | Self-assembly (thermal gelation > 37 °C), photo crosslinking | Extrusion | 20% (17% pure F-127, 3% acrylated F-127) | 86.3% (Day 14) | Chondrocyte culturing [70]. | 70 |
| Silicone | Chemical crosslinking | Inkjet, extrusion | Commercially available silicone (Ecoflex 50, Ecoflex30) | High (120 h) | Meniscus implants [71]. | 71 |
| Composite | Bioink Formulation Techniques | Printing Method | Optimal Polymer Concentration | Cell Viability | Application | Ref. |
|---|---|---|---|---|---|---|
| Collagen–gold nanowires (GNWs) | Self-assembly after neutralization (collagen) + genipin (GNWs). | Extrusion | 5% Collagen | >90% (21 days) | Muscle tissue repair | 109 |
| Agarose– Laponite | Mixing in distilled water + autoclaving (115 °C). | Extrusion | 3% Agarose; 2–3% Laponite | High (7 days) | Fibroblast culturing | 82 |
| Alginate– poloxamer | Self-assembly above 37 °C (Pluronic) + divalent ions (alginate). | Extrusion | 6% Alginate; 13% Poloxamer (F-127) | 83 ± 6% (Day 7), full-sized tracheal ring (Day 35) | Cartilage formation | 111 |
| PVA–chitosan (CS) | Mixing in distilled water (PVA) + dissolution in acetic acid and distilled water (CS). | Extrusion | 13% PVA; 1, 3 or 5% Chitosan | 80–90% (7 days) | Bioprinted cornea | 112 |
| Silk–gelatin | Dissolution in 37 °C, enzymatic crosslinking (mushroom tyrosinase). | Extrusion | 5% Silk; 5% Gelatin | 96% (>28 days) | Skin bioprinting | 113 |
| Matrigel–agarose | Thermal self- assembly (4 °C Matrigel, 37 °C agarose). | Extrusion | 50% Matrigel; 3% Agarose | 77% (6 days) | Intestinal model | 114 |
| Cellulose–carbon nanotubes | Aqueous dispersion with NaOH | Extrusion | 2% Cellulose; 2% CNT | High | Neural development | 115 |
| GelMA–chitosan-dextran | UV, Irgacure (GelMA) + PBS dissolution (chitosan, dextran) | Extrusion | 13% GelMA; Chitosan-Dextran 8% | High | Wound healing | 116 |
| Collagen–GelMA | Enzymatic tyrosinase (collagen) + UV, Irgacure (GelMA). | Extrusion | 8% Collagen; 5% GelMA | 94% (14 days) | Skin wound repair | 117 |
| HA–Sodium Alginate (SA)–Gelatin (GA) | Homogeneous blending in deionized water (HA, SA, gelatin) + CaCl2 (SA). | Extrusion | 2% HA; 1% SA; 7.5 % GA | 85% (14 days) | Brain microenvironment mimetic model | 118 |
| Silk Composites | Grinding silk nanofibers dispersed in water and adding to the solution of composites such as HA, PVA and chitosan. | Extrusion | 1% | 92% (6 days) | Fibroblast culturing, anatomical model printing (ear, nose) | 119 |
| Categories | Perfusion Channels [40] | Perfluorocarbons (PFCs) [41] | Peroxides [42] |
|---|---|---|---|
| General Description | Uses microfluidics to construct perfusable networks within printed constructs. | PFCs are non-toxic, chemically inert, immiscible fluids with high oxygen and carbon dioxide transportability. | Peroxides are oxygen generators upon ready decomposition. |
| Mechanism of oxygenation | The perfusable channels are made of sacrificial materials to allow for mass O2 and nutrient exchange and later for guided development of blood vessels. | PFCs are hydrocarbon structures having fluorine or halogen substitutes in place of hydrogen within the polymer backbone. Being an electron-acceptor, fluorine can dissolve gases (e.g., O2) through diffusion. | Peroxides can interact with water to undergo hydrolytic decomposition and produce oxygen. |
| Requirements | Co-axial printing to allow simultaneous deposition of the structural bioink and the sacrificial template. | Being extremely hydrophobic with certain lipophilic characteristics, PFCs require surfactants such as lecithin to form suitable emulsions that can be incorporated within the bioink. | Peroxide decomposition into water and oxygen is related to the formation of hydrogen peroxide that is detrimental to cells. The incorporation of catalase enzyme within the printed bioink, along with the oxygen-generating peroxides. |
| Need | Inducement of angiogenesis through growth factors requires long intervals to establish functional vasculature during which mass transport may be compromised due to diffusion limitation in the thicker structures [12]. Pre-vascularization offers immediate oxygen and nutrient perfusion, by-passing the time lag associated with vasculature formation. | In view of the high molecular ratios of dissolved O2 in PFC (5O2:1PFC), 1000-times higher than water, the incorporation of PFCs within bioinks can help attract and direct oxygen from the growth medium to the cells encapsulated within the printed construct to better oxygenate the cells. | Incorporation of peroxides within bioinks can assist in timely decomposition of hydrogen peroxide decomposition and maintain cell viability within the printed construct (as seen in the pictorial representation below) [134]. |
| Examples | Sacrificial channels can be made from temperature-sensitive biomaterials such as gelatin, GelMA, or Pluronic. | Perfluoro-octyl bromide (PFOB) and perfluoro-decalin (PFD) can be used. | Calcium, magnesium, or sodium peroxides can be used. |
| Efficiency | Co-fabrication of perfusable vascular channels has seen improved cell survival (maintained 80% viability over a 14-day period) and function within the printed structures [40]. | Molecular ratios of dissolved O2 are 1O2:200water in water, but 5O2:1PFD in PFD, resulting in a 1000-times increased molecular solubility of O2 for PFD compared to water (as seen in pictorial representation below). | Cell viability analysis on day 7 after incorporating 1% calcium peroxide is 80% [134]. |
| Pictorial Representation |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parimala Chelvi Ratnamani, M.; Zhang, X.; Wang, H. A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting. Gels 2022, 8, 239. https://doi.org/10.3390/gels8040239
Parimala Chelvi Ratnamani M, Zhang X, Wang H. A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting. Gels. 2022; 8(4):239. https://doi.org/10.3390/gels8040239
Chicago/Turabian StyleParimala Chelvi Ratnamani, Matangi, Xinping Zhang, and Hongjun Wang. 2022. "A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting" Gels 8, no. 4: 239. https://doi.org/10.3390/gels8040239
APA StyleParimala Chelvi Ratnamani, M., Zhang, X., & Wang, H. (2022). A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting. Gels, 8(4), 239. https://doi.org/10.3390/gels8040239







