Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Materials
2.3. Nanogel Synthesis and Characterization
2.3.1. Synthesis of CH/HA Nanogels
2.3.2. Nanogel Purification and Lyophilization
2.3.3. CH/HA Nanogel Characterization
2.4. Cells Studies
2.4.1. Culture of Chondrocytes, Synoviocytes and Osteoblasts
2.4.2. MTS and LDH Assays
2.4.3. Nitric Oxide Quantification
2.4.4. DNA Extraction
2.5. Zebrafish Embryo Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nanogel Synthesis: Variability Factors and Resulting Physicochemical Properties
3.1.1. Optimization of the Formulation of CH-Based NG
3.1.2. Effect of the Acidic Solution
3.2. Establishing Biocompatibility for CH-Based Nanogels
3.2.1. Purified CH-Based Nanogels Were Not Cytotoxic nor Generate Nitric Oxide to Osteocartilaginous Cells
3.2.2. Nanogels Were Moderately Genotoxic in a Dose-Dependent Manner but Did Not Significantly Induce Acute Embryotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schurman, D.J.; Smith, R.L. Osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S183–S189. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Brown, S.; Kumar, S.; Sharma, B. Intra-Articular Targeting of Nanomaterials for the Treatment of Osteoarthritis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef]
- Rai, M.F.; Pham, C.T. Intra-Articular Drug Delivery Systems for Joint Diseases. Curr. Opin. Pharmacol. 2018, 40, 67–73. [Google Scholar] [CrossRef]
- Wehling, P.; Evans, C.; Wehling, J.; Maixner, W. Effectiveness of Intra-Articular Therapies in Osteoarthritis: A Literature Review. Ther. Adv. Musculoskelet. Dis. 2017, 9, 183–196. [Google Scholar] [CrossRef]
- Sayed Aly, M. Intra-Articular Drug Delivery: A Fast Growing Approach. Recent Pat. Drug Deliv. Formul. 2008, 2, 231–237. [Google Scholar] [CrossRef]
- Edwards, S.H.R. Intra-Articular Drug Delivery: The Challenge to Extend Drug Residence Time within the Joint. Vet. J. 2011, 190, 15–21. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI Guidelines for the Non-Surgical Management of Knee Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- Grazina, R.; Andrade, R.; Bastos, R.; Costa, D.; Pereira, R.; Marinhas, J.; Maestro, A.; Espregueira-Mendes, J. Clinical Management in Early OA. In Advances in Experimental Medicine and Biology; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; Volume 1059, pp. 111–135. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Agarwal, R.; Diaz-Ruiz, C.A.; Willett, N.J.; Wang, P.; Lee, L.A.; Wang, Q.; Guldberg, R.E.; García, A.J. Nanoengineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins. Adv. Healthc. Mater. 2014, 3, 1562–1567. [Google Scholar] [CrossRef]
- Kang, M.L.; Ko, J.-Y.; Kim, J.E.; Im, G.-I. Intra-Articular Delivery of Kartogenin-Conjugated Chitosan Nano/Microparticles for Cartilage Regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. In Recent Advances in Biopolymers; InTech: London, UK, 2016. [Google Scholar]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of Polymer-Based Biomaterials and Medical Devices—Regulations, in Vitro Screening and Risk-Management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-Engineered Intra-Articular Drug Delivery Systems for Osteoarthritis Therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, B.; Hu, C.; Zhao, J. An Overview of Hydrogel-Based Intra-Articular Drug Delivery for the Treatment of Osteoarthritis. Colloids Surf. B Biointerfaces 2017, 154, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Peck, Y.; Lau, J.E.J.; Hee, H.T.; Wang, D.-A. Hydrogel Based Cartilaginous Tissue Regeneration: Recent Insights and Technologies. Biomater. Sci. 2017, 5, 613–631. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017, 8, 204173141772646. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J. Viscosupplementation for Osteoarthritis of the Knee. N. Engl. J. Med. 2015, 372, 1040–1047. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1077, pp. 475–485. ISBN 9789811309472. [Google Scholar]
- Mohammadinejad, R.; Ashrafizadeh, M.; Pardakhty, A.; Uzieliene, I.; Denkovskij, J.; Bernotiene, E.; Janssen, L.; Lorite, G.S.; Saarakkala, S.; Mobasheri, A. Nanotechnological Strategies for Osteoarthritis Diagnosis, Monitoring, Clinical Management, and Regenerative Medicine: Recent Advances and Future Opportunities. Curr. Rheumatol. Rep. 2020, 22, 12. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, I.; Weinberg, G.L. Nanomedicines for Chronic Non-Infectious Arthritis: The Clinician’s Perspective. Nanomed. Nanotechnol. Biol. Med. 2012, 8, S77–S82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew. Chem. Int. Ed. 2012, 51, 9119–9122. [Google Scholar] [CrossRef]
- Callewaert, M.; Roullin, V.G.; Cadiou, C.; Millart, E.; Van Gulik, L.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; Laurent, S.; Elst, L.V.; et al. Tuning the Composition of Biocompatible Gd Nanohydrogels to Achieve Hypersensitive Dual T1/T2 MRI Contrast Agents. J. Mater. Chem. B 2014, 2, 6397–6405. [Google Scholar] [CrossRef] [PubMed]
- Manacu, C.A.; Martel-Pelletier, J.; Roy-Beaudry, M.; Pelletier, J.-P.; Fernandes, J.C.; Shipkolye, F.S.; Mitrovic, D.R.; Moldovan, F. Endothelin-1 in Osteoarthritic Chondrocytes Triggers Nitric Oxide Production and Upregulates Collagenase Production. Arthritis Res. Ther. 2005, 7, R324–R332. [Google Scholar] [CrossRef] [Green Version]
- Roy-Beaudry, M.; Martel-Pelletier, J.; Pelletier, J.-P.; M’Barek, K.N.; Christgau, S.; Shipkolye, F.; Moldovan, F. Endothelin 1 Promotes Osteoarthritic Cartilage Degradation via Matrix Metalloprotease 1 and Matrix Metalloprotease 13 Induction. Arthritis Rheum. 2003, 48, 2855–2864. [Google Scholar] [CrossRef]
- Moldovan, F.; Pelletier, J.-P.; Hambor, J.; Cloutier, J.-M.; Martel-Pelletier, J. Collagenase-3 (Matrix Metalloprotease 13) Is Preferentially Localized in the Deep Layer of Human Arthritic Cartilage in Situ. In Vitro Mimicking Effect by Transforming Growth Factor β. Arthritis Rheum. 1997, 40, 1653–1661. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Villegas-Peralta, Y.; López-Cervantes, J.; Madera Santana, T.J.; Sánchez-Duarte, R.G.; Sánchez-Machado, D.I.; del Rosario Martínez-Macías, M.; Correa-Murrieta, M.A. Impact of the Molecular Weight on the Size of Chitosan Nanoparticles: Characterization and Its Solid-State Application. Polym. Bull. 2021, 78, 813–832. [Google Scholar] [CrossRef]
- Bruinsmann, F.; Pigana, S.; Aguirre, T.; Souto, G.; Pereira, G.; Bianchera, A.; Fasiolo, L.; Colombo, G.; Marques, M.; Pohlmann, A.; et al. Chitosan-Coated Nanoparticles: Effect of Chitosan Molecular Weight on Nasal Transmucosal Delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.-L.; Vo, T.P.K.; Nguyen, A.D. Preparation of Chitosan Nanoparticles by TPP Ionic Gelation Combined with Spray Drying, and the Antibacterial Activity of Chitosan Nanoparticles and a Chitosan Nanoparticle–Amoxicillin Complex. Res. Chem. Intermed. 2017, 43, 3527–3537. [Google Scholar] [CrossRef]
- Lallana, E.; Rios de la Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Jamil, S.N.A.M. Synthesis and Optimization of Chitosan Nanoparticles Loaded with L-Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [Green Version]
- FDA. Guide to Inspections—Lyophilization of Parenterals. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/lyophilization-parenteral-793 (accessed on 18 May 2021).
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Singh, A.; Castillo, H.A.; Brown, J.; Kaslin, J.; Dwyer, K.M.; Gibert, Y. High Glucose Levels Affect Retinal Patterning during Zebrafish Embryogenesis. Sci. Rep. 2019, 9, 4121. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef]
- Wu, Q.-X.; Lin, D.-Q.; Yao, S.-J. Design of Chitosan and Its Water Soluble Derivatives-Based Drug Carriers with Polyelectrolyte Complexes. Mar. Drugs 2014, 12, 6236–6253. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C. Preparation and Properties of Ionically Cross-Linked Chitosan Nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Gheran, C.; Rigaux, G.; Callewaert, M.; Berquand, A.; Molinari, M.; Chuburu, F.; Voicu, S.; Dinischiotu, A. Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging. Nanomaterials 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation Mechanism of Monodisperse, Low Molecular Weight Chitosan Nanoparticles by Ionic Gelation Technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In Vitro Blood Cell Viability Profiling of Polymers Used in Molecular Assembly. Sci. Rep. 2017, 7, 9481. [Google Scholar] [CrossRef] [PubMed]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of Polycations: Relationship of Molecular Weight and the Hydrolytic Theory of the Mechanism of Toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondon, E.P.; Benabdoun, H.A.; Vallières, F.; Segalla Petrônio, M.; Tiera, M.J.; Benderdour, M.; Fernandes, J.C. Evidence Supporting the Safety of Pegylated Diethylaminoethyl-Chitosan Polymer as a Nanovector for Gene Therapy Applications. Int. J. Nanomed. 2020, 15, 6183–6200. [Google Scholar] [CrossRef]
- Arca, H.Ç.; Şenel, S. Chitosan Based Systems for Tissue Engineering Part II: Soft Tissues. Fabad J. Pharm. Sci. 2008, 33, 211–216. [Google Scholar]
- Ribeiro, J.C.V.; Vieira, R.S.; Melo, I.M.; Araújo, V.M.A.; Lima, V. Versatility of Chitosan-Based Biomaterials and Their Use as Scaffolds for Tissue Regeneration. Sci. World J. 2017, 2017, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, F.; Oliveira Formiga, R.; Fernandes da Silva Coimbra, J.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. Targeting Nitric Oxide as a Key Modulator of Sepsis, Arthritis and Pain. Nitric Oxide Biol. Chem. 2019, 89, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin Inhibits IL-1β-Induced Inflammation in Rat Chondrocytes and Attenuates Osteoarthritis Progression in a Rat Model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC Exosomes Alleviate Temporomandibular Joint Osteoarthritis by Attenuating Inflammation and Restoring Matrix Homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Deng, R.; Xia, G.; Shang, X.; Zhou, P. Hyaluronic Acid-Chitosan Nanoparticles Encoding CrmA Attenuate Interleukin-1β Induced Inflammation in Synoviocytes In vitro. Int. J. Mol. Med. 2018, 43, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.-H.; Qiu, B.; Zhou, P.-H. Chitosan/Hyaluronic Acid/Plasmid-DNA Nanoparticles Encoding Interleukin-1 Receptor Antagonist Attenuate Inflammation in Synoviocytes Induced by Interleukin-1 Beta. J. Mater. Sci. Mater. Med. 2018, 29, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.-D.; Zhao, H.-Q.; Wang, K.; Lv, L.-L. Novel Hyaluronic Acid–Chitosan Nanoparticles as Non-Viral Gene Delivery Vectors Targeting Osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Wang, H.; Jreyssaty, C.; Benderdour, M.; Lavigne, P.; Qiu, X.; Winnik, F.M.; Zhang, X.; Dai, K.; Shi, Q. Bone-Protective Effects of Nonviral Gene Therapy With Folate–Chitosan DNA Nanoparticle Containing Interleukin-1 Receptor Antagonist Gene in Rats With Adjuvant-Induced Arthritis. Mol. Ther. 2008, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Alradwan, I.; Majrashi, M.A.; Alsaffar, B.A.; Algarni, A.T.; Alsuabeyl, M.S.; Alrabiah, H.; Tirelli, N.; Alhasan, A.H. Cellular Responses of Hyaluronic Acid-Coated Chitosan Nanoparticles. Toxicol. Res. 2018, 7, 942–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.L.; Lavertu, M.; Winnik, F.M.; Buschmann, M.D. Stability and Binding Affinity of DNA/Chitosan Complexes by Polyanion Competition. Carbohydr. Polym. 2017, 176, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Anaya, L.M.; Fernández-Solís, K.G.; Rosselgong, J.; Nano-Rodríguez, J.L.E.; Carvajal, F.; Rinaudo, M. Chitosan-DNA Polyelectrolyte Complex: Influence of Chitosan Characteristics and Mechanism of Complex Formation. Int. J. Biol. Macromol. 2019, 126, 1037–1049. [Google Scholar] [CrossRef]
- Jia, H.-R.; Zhu, Y.-X.; Duan, Q.-Y.; Chen, Z.; Wu, F.-G. Nanomaterials Meet Zebrafish: Toxicity Evaluation and Drug Delivery Applications. J. Control. Release 2019, 311–312, 301–318. [Google Scholar] [CrossRef]
- Haque, E.; Ward, A.C. Zebrafish as a Model to Evaluate Nanoparticle Toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Rabanel, J.M.; Piec, P.A.; Landri, S.; Patten, S.A.; Ramassamy, C. Transport of PEGylated-PLA Nanoparticles across a Blood Brain Barrier Model, Entry into Neuronal Cells and in Vivo Brain Bioavailability. J. Control. Release 2020, 328, 679–695. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together. BioMed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bergen, D.J.M.; Kague, E.; Hammond, C.L. Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds. Front. Endocrinol. 2019, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Lleras-Forero, L.; Winkler, C.; Schulte-Merker, S. Zebrafish and Medaka as Models for Biomedical Research of Bone Diseases. Dev. Biol. 2020, 457, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and Toxicology Evaluation of Chitosan Nanoparticles on the Embryonic Development of Zebrafish, Danio Rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Wang, Q.; Han, F.; Shao, J.-Z.; Gao, J.-Q. Toxicity Evaluation of Biodegradable Chitosan Nanoparticles Using a Zebrafish Embryo Model. Int. J. Nanomed. 2011, 6, 3351. [Google Scholar] [CrossRef] [Green Version]
- Abou-Saleh, H.; Younes, N.; Rasool, K.; Younis, M.; Prieto, R.; Yassine, H.; Mahmoud, K.; Pintus, G.; Nasrallah, G. Impaired Liver Size and Compromised Neurobehavioral Activity Are Elicited by Chitosan Nanoparticles in the Zebrafish Embryo Model. Nanomaterials 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, N.; Pintus, G.; Al-Asmakh, M.; Rasool, K.; Younes, S.; Calzolari, S.; Mahmoud, K.A.; Nasrallah, G.K. “Safe” Chitosan/Zinc Oxide Nanocomposite Has Minimal Organ-Specific Toxicity in Early Stages of Zebrafish Development. ACS Biomater. Sci. Eng. 2020, 6, 38–47. [Google Scholar] [CrossRef]
- Chou, C.-M.; Mi, F.-L.; Horng, J.-L.; Lin, L.-Y.; Tsai, M.-L.; Liu, C.-L.; Lu, K.-Y.; Chu, C.-Y.; Chen, Y.-T.; Lee, Y.-L.A.; et al. Characterization and Toxicology Evaluation of Low Molecular Weight Chitosan on Zebrafish. Carbohydr. Polym. 2020, 240, 116164. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I.; Levašič, V.; Vidmar, J.; Kovač, S.; Trebše, R. PH and Metal Concentration of Synovial Fluid of Osteoarthritic Joints and Joints with Metal Replacements. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2507–2515. [Google Scholar] [CrossRef]
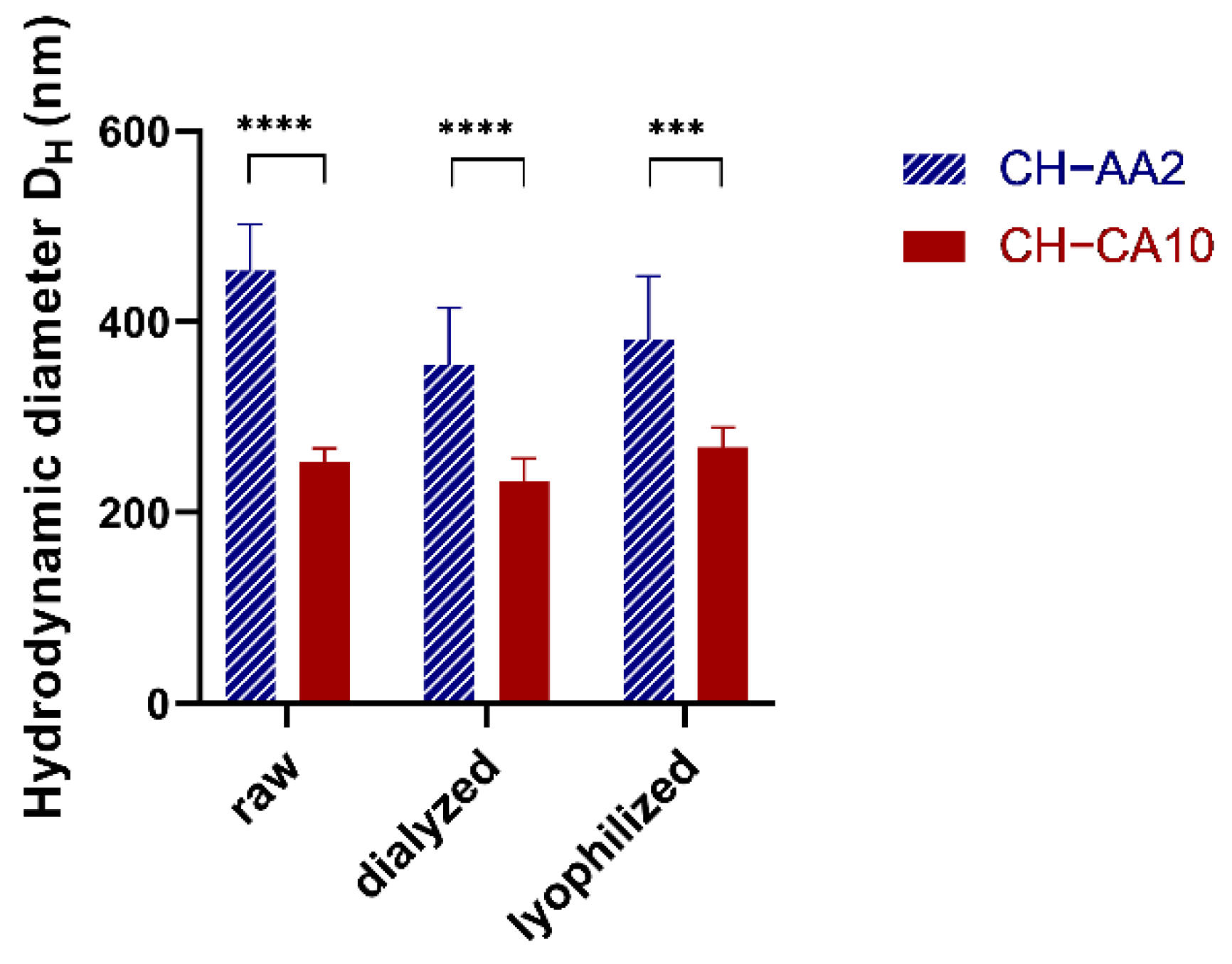



| NG CH−AA2 | NG CH−CA10 | |||||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | ZP (mV) | pH | Size (nm) | PdI | ZP (mV) | pH | |
| raw | 453 ± 49 | 0.28 ± 0.03 | 49 ± 9 | 3.31 ± 0.02 | 252 ± 15 | 0.19 ± 0.02 | 27 ± 3 | 1.93 ± 0.02 |
| dialyzed | 355 ± 60 | 0.32 ± 0.02 | 40 ± 8 | 5.81 ± 0.09 | 233 ± 24 | 0.24 ± 0.03 | 30 ± 7 | 4.21 ± 0.12 |
| lyophilized | 382 ± 92 | 0.33 ± 0.02 | 40 ± 10 | 5.79 ± 0.11 | 268 ± 21 | 0.26 ± 0.03 | 27 ± 8 | 3.88 ± 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manivong, S.; Garcia Ac, A.; Patten, S.A.; Fernandes, J.C.; Benderdour, M.; Banquy, X.; Moldovan, F.; Roullin, V.G. Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials 2022, 12, 1337. https://doi.org/10.3390/nano12081337
Manivong S, Garcia Ac A, Patten SA, Fernandes JC, Benderdour M, Banquy X, Moldovan F, Roullin VG. Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials. 2022; 12(8):1337. https://doi.org/10.3390/nano12081337
Chicago/Turabian StyleManivong, Seng, Araceli Garcia Ac, Shunmoogum A. Patten, Julio C. Fernandes, Mohamed Benderdour, Xavier Banquy, Florina Moldovan, and Valérie Gaëlle Roullin. 2022. "Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints" Nanomaterials 12, no. 8: 1337. https://doi.org/10.3390/nano12081337
APA StyleManivong, S., Garcia Ac, A., Patten, S. A., Fernandes, J. C., Benderdour, M., Banquy, X., Moldovan, F., & Roullin, V. G. (2022). Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials, 12(8), 1337. https://doi.org/10.3390/nano12081337





