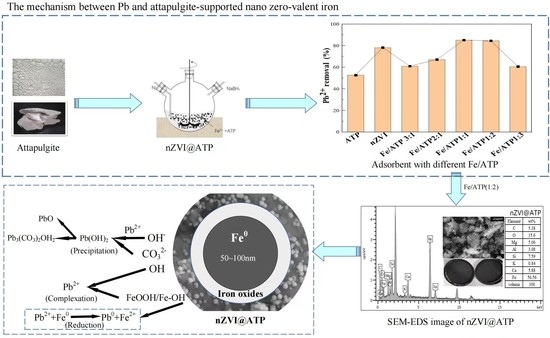
Mechanism-Enhanced Active Attapulgite-Supported Nanoscale Zero-Valent Iron for Efficient Removal of Pb2+ from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of nZVI@ATP
2.3. Characterization of Adsorbents
2.4. Batch Experiments
3. Results and Discussion
3.1. Characterizations of Materials
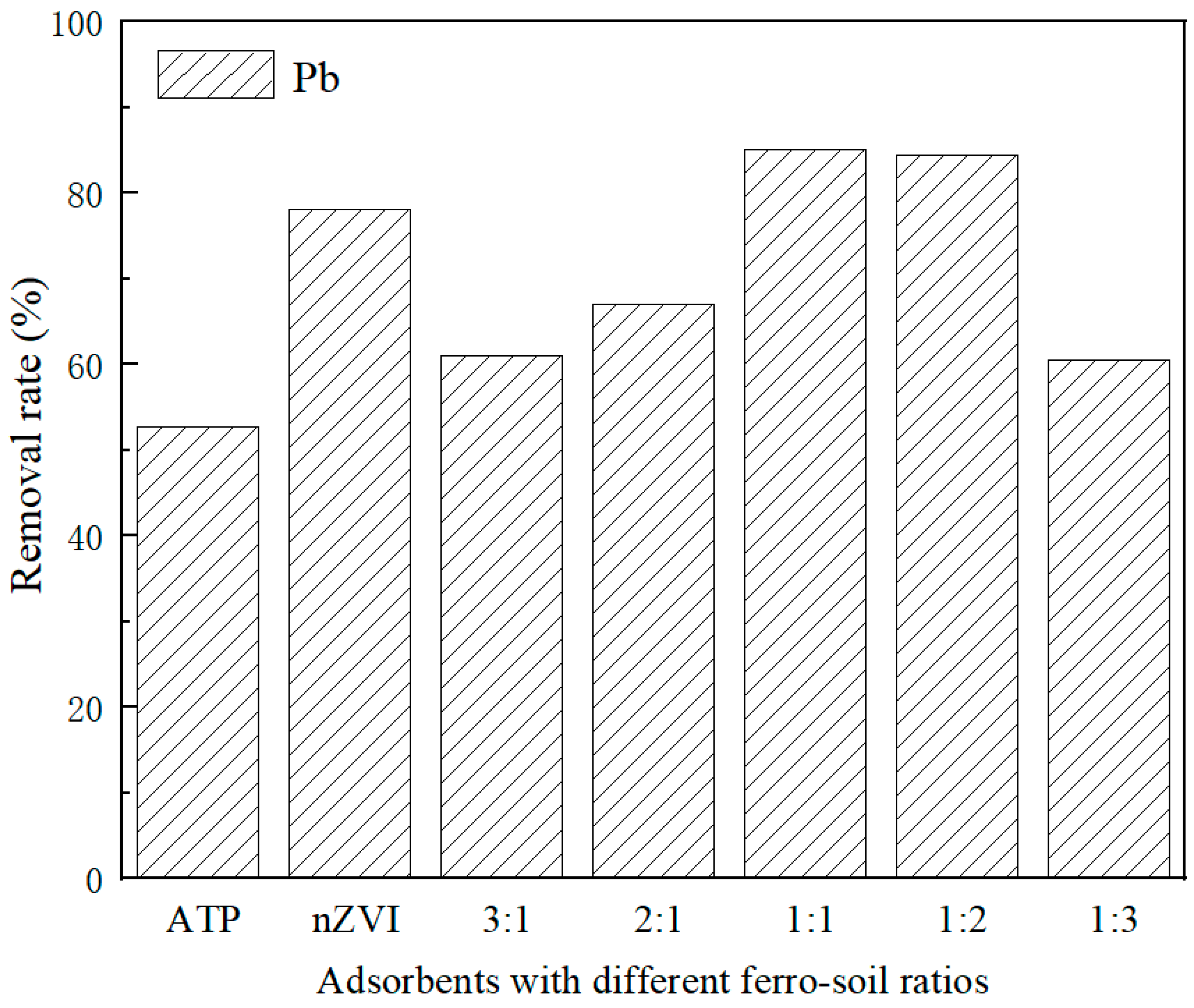
3.2. Effect of Different Materials and Nanoscale Zero-Valent Iron Loading on Pb2+ Removal
3.3. Sorption Kinetics and Isotherms (Adsorption Properties)
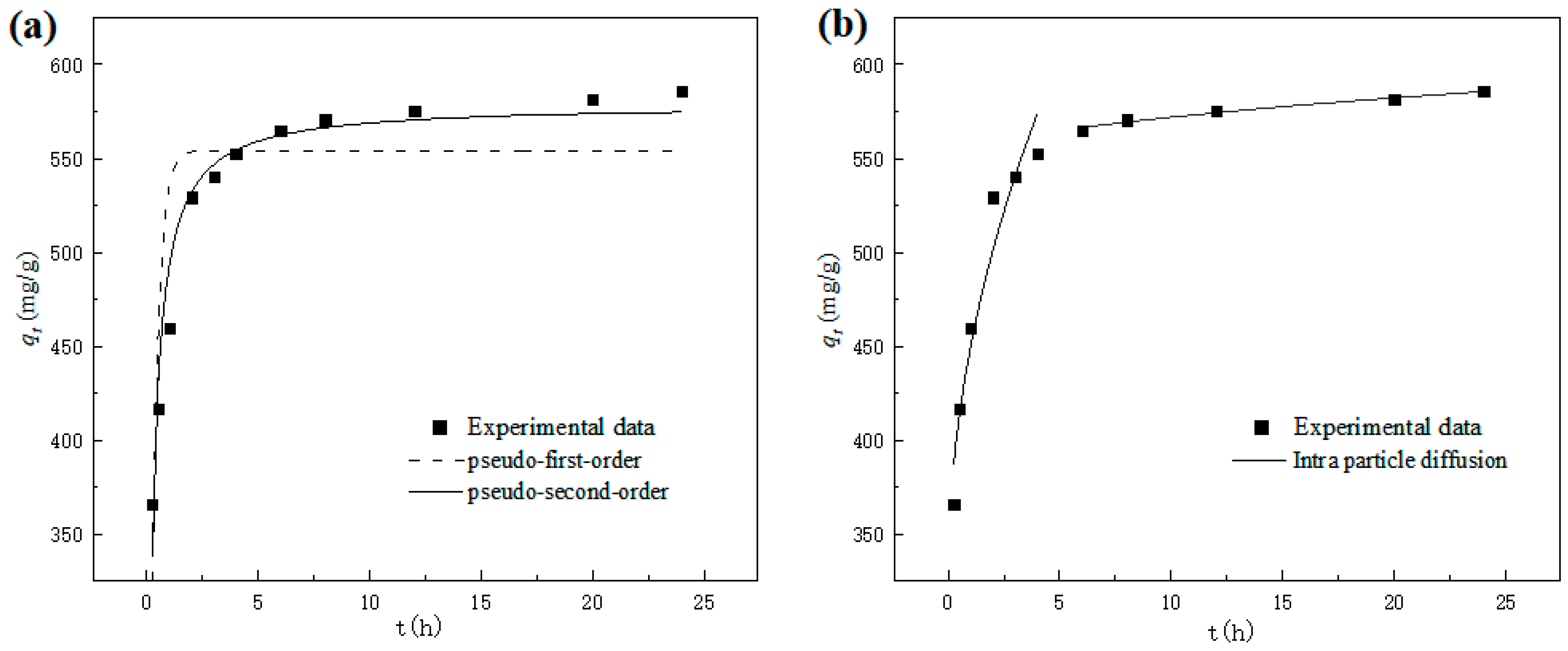
3.3.1. Sorption Kinetics
3.3.2. Adsorption Isotherms and Thermodynamic
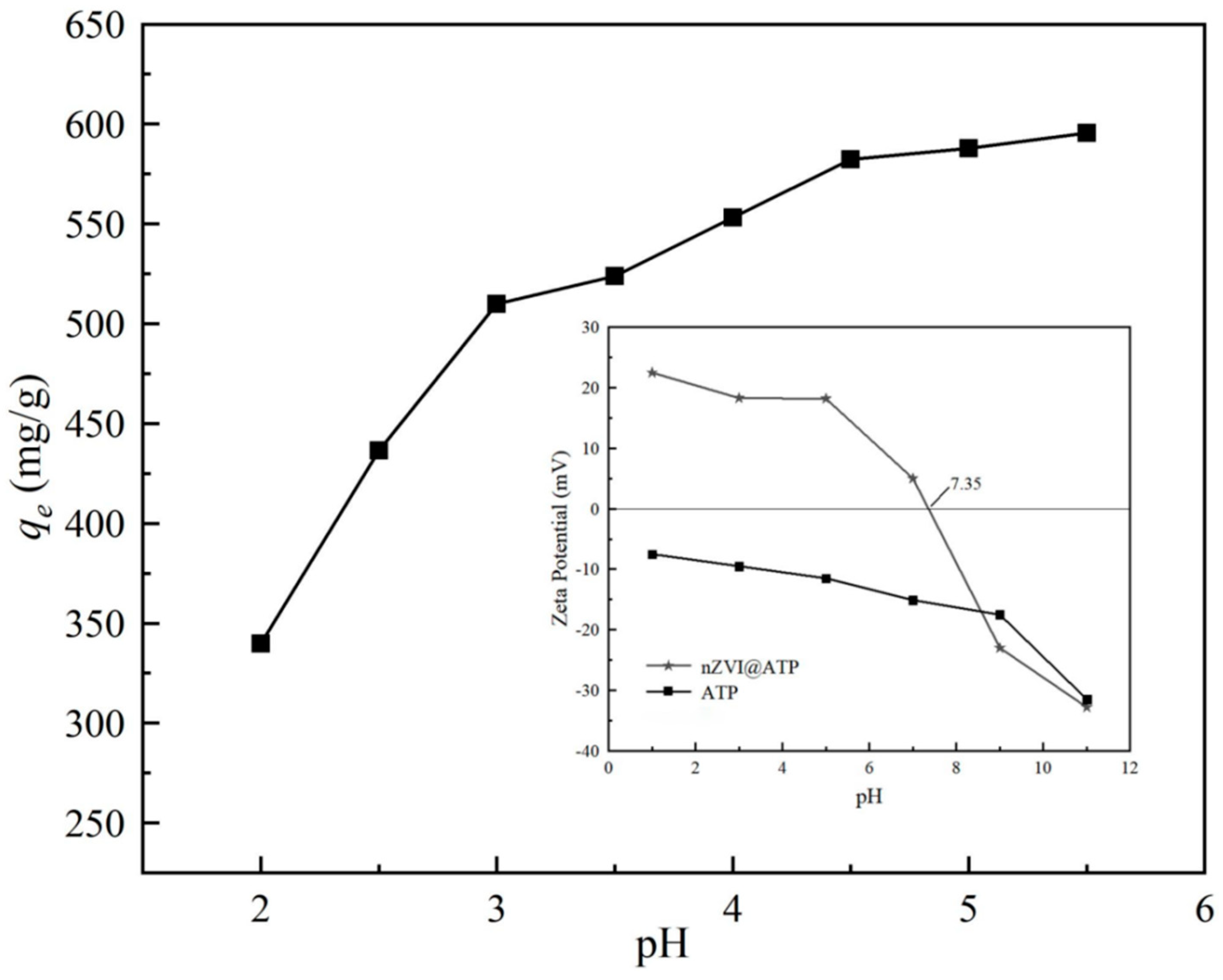
3.4. Effects of Solution pH on Pb2+ Removal Capacity
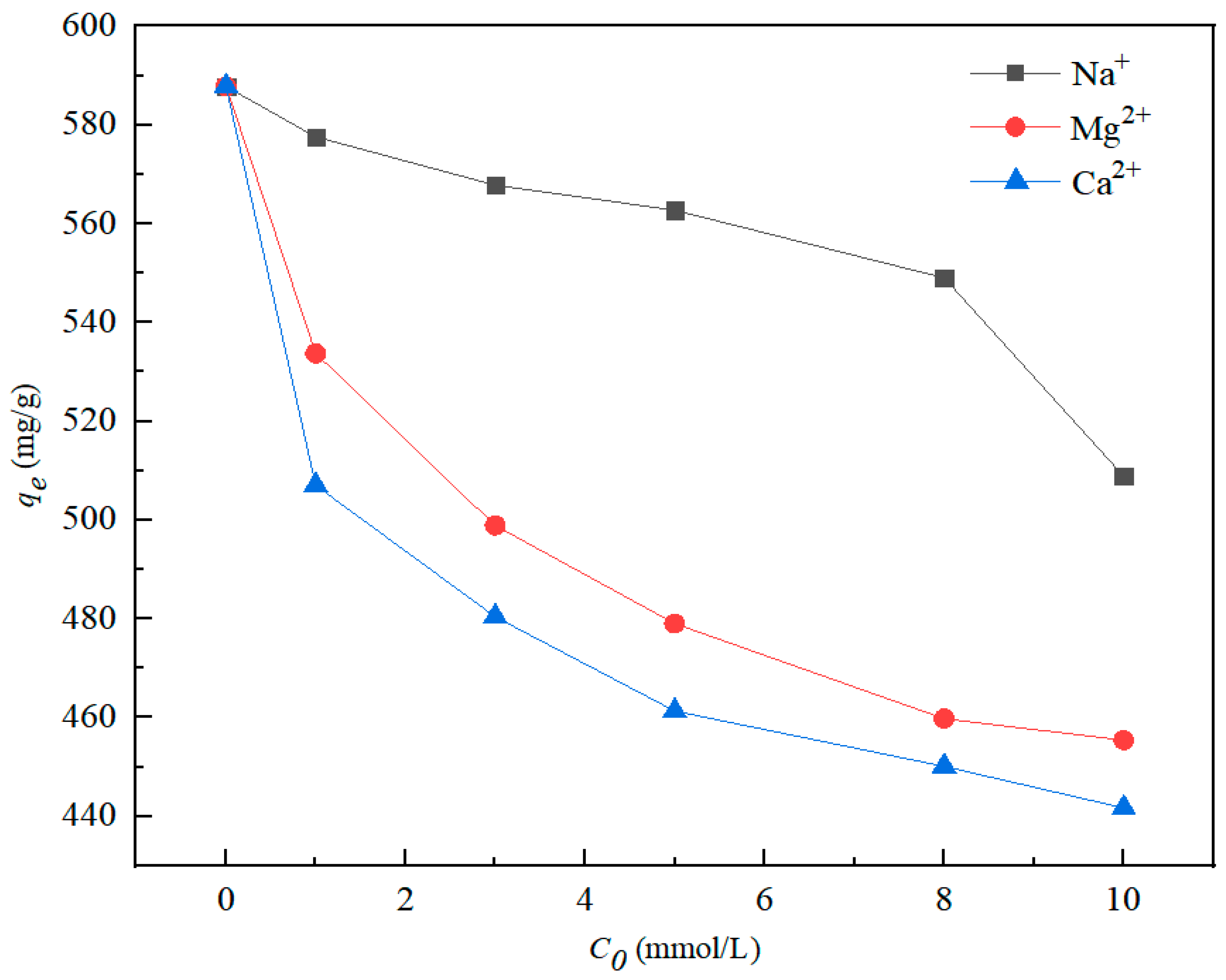
3.5. Influence of Coexisting Cations
3.6. Pb2+ Removal of Mechanisms by nZVI@ATP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiyadh, S.S.; Alsaadi, M.A.; Jaafar, W.Z.; Alomar, M.K.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; EI-Shafie, A. Review on Heavy Metal Adsorption Processes By Carbon Nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hou, D.; Zhang, P.; Wang, Y.; Zhang, Y.; Shi, P.; O’Connor, D. Lead based paint in children’s toys sold on China’s major online shopping platforms. Environ. Pollut. 2018, 241, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.X.; Zhao, G.X.; Chen, C.L.; Chai, A.F.; Alsaedi, A.; Hayat, T.; Wang, X.T. Metal-organic Framework-based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Freeman, J.L. Zebrafish as a Model for Investigating Developmental Lead (Pb) Neurotoxicity as a Risk Factor in Adult Neurodegenerative Disease: A Mini-review. Neurotoxicology 2014, 43, 57–64. [Google Scholar] [CrossRef]
- Guha, T.; Barman, S.; Mukherjee, A.; Kundu, R. Nano-scale zero valent iron modulates Fe/Cd transporters and immobilizes soil Cd for production of Cd free rice. Chemosphere 2020, 260, 127533. [Google Scholar] [CrossRef]
- Gomes, H.I.; Fan, G.; Mateus, E.P.; Dias-Ferreira, C.; Ribeiro, A.B. Assessment of Combined Electro–nanoremediation of Molinate Contaminated Soil. Sci. Total Environ. 2014, 493, 178–184. [Google Scholar] [CrossRef]
- Visentin, C.; Trentin, A.S.; Braun, A.B.; Thome, A. Lifecycle Assessment of Environmental and Economic Impacts of Nano-iron Synthesis Process for Application in Contaminated Site Remediation. J. Clean. Prod. 2019, 231, 307–319. [Google Scholar] [CrossRef]
- Bae, S.; Collins, R.N.; Waite, T.D.; Hanna, K. Advances in surface passivation of nanoscale zerovalent iron: A critical review. Environ. Sci. Technol. 2018, 52, 12010–12025. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, W.; Hu, N.; Gu, T.; Ling, L.; Zhang, W.X. Enrichment of uranium from wastewater with nanoscale zero-valent iron (nZVI). Environ. Sci. Nano 2021, 8, 666–674. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, W.D.Y. An Overview of Preparation and Applications of Stabilized Zero-valent Iron Nanoparticles for Soil and Groundwater Remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocur, C.M.; Sleep, B.E.; O’carroll, D.M. Moving Into the Third Decade of Nanoscale zero-valent Iron (nZVI) Development: Best Practices for Field Implementation. In Nanoscale Zerovalent Iron Particles for Environmental Restoration; Springer: Cham, Switzerland, 2019; pp. 293–333. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhao, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, W.Q.; Igalavithana, A.D.; Oleszczuk, P.; Ok, Y.S.; et al. Biochar-supported nZVI (nZVI/BC) for Contaminant Removal from Soil and Water: A Critical Review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Guan, J.; Li, Z.X.; Chen, S.; Gu, W.X. Zero-valent iron supported on expanded graphite from spent lithium-ion battery anodes and ferric chloride for the degradation of 4-chlorophenol in water. Chemosphere 2022, 290, 133381. [Google Scholar] [CrossRef]
- Selvan, B.K.; Thiyagarajan, K.; Das, S.; Jaya, N.; Jabasingh, S.A.; Saravanan, P.; Rajasimman, M.; Vasseghian, Y. Synthesis and characterization of nano zerovalent iron-kaolin clay (nZVI-Kaol) composite polyethersulfone (PES) membrane for the efficacious As2O3 removal from potable water samples. Chemosphere 2022, 288, 132405. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.M.; Ma, K.X.; Qin, Q.D. Facile synthesis of high iron content activated carbon-supported nanoscale zero-valent iron for enhanced Cr(VI) removal in aqueous solution. Chemosphere 2022, 291, 132709. [Google Scholar] [CrossRef]
- Boudriche, L.; Calvet, R.; Hamdi, B.; Balard, H. Surface properties evolution of attapulgite by IGC analysis as a function of thermal treatment. Colloids Surf. A. 2012, 399, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.B.; Kong, M.; Gu, X.H.; Chen, H. Removal of Arsenic from Water by Porous Charred Granulated Attapulgite-supported Hydrated Iron Oxide in Bath and Column Modes. J. Clean. Prod. 2017, 166, 88–97. [Google Scholar] [CrossRef]
- Yin, H.B.; Zhu, J.C. In Situ Remediation of Metal Contaminated Lake Sediment Using Naturally Occurring, Calcium-rich Clay Mineral-based Low-cost Amendment. Chem. Eng. J. 2016, 285, 112–120. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, J.G.; Tang, Y.; Zhang, Z. Selective Adsorption of Pb2+ and Cu2+ on Amino-modified Attapulgite: Kinetic, Thermal Dynamic and DFT Studies. J. Hazard. Mater. 2021, 404, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Lin, Q.; Zhong, Q.; Zhang, X.; Wen, X.; Luo, H. Removal of Cd(II) and Pb(II) from Aqueous Solution by Modified Attapulgite Clay. Arab. J. Chem. 2020, 13, 4994–5008. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of Sludge Based Adsorbents for the Removal of Various Pollutants: A Review. Sci. Total Environ. 2016, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Islam, M.S.; Wang, S.Y.; Messele, S.A.; Naeth, M.A.; EI-Din, M.G.; Chang, S.X. Biochar Properties and Lead(II) Adsorption Capacity Depend on Feedstock Type, Pyrolysis Temperature, and Steam Activation. Chemosphere 2019, 231, 393–404. [Google Scholar] [CrossRef]
- Trazzi, P.A.; Leahy, J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and desorption of phosphate on biochar. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Arshadi, M.; Soleymanzadeh, M.; Salvacion, J.; SalimiVahid, F. Nanoscale zerovalent iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: Kinetics, thermodynamic and mechanism. J. Colloid Interf. Sci. 2014, 426, 241–251. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.; Santos, M.; Oliveira, M.; Carvalho, M.; Osajima, J.A.; Filho, E. Effects of Acid Treatment on the Clay Palygorskite: XRD, Surface Area, Morphological and Chemical Composition. Mater. Res. Bull. 2014, 17, 3–8. [Google Scholar] [CrossRef]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.J.; Park, Y.J.; Shea, P.J.; Lee, W.H.; Kim, H.M.; Oh, B.T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, S.; Lu, X.Q.; Chen, Z.L. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D. Fabrication and Characterization of Hydrophilic Corn Stalk Biochar-supported Nanoscale zero-valent Iron Composites for Efficient Metal Removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.J.; Li, T.; Zhou, C. Physicochemical studies toward the removal of Zn( II ) and Pb( II ) ions through adsorption on montmorillonite-supported zero-valent ironnanoparticles. RSC Adv. 2015, 5, 29859–29871. [Google Scholar] [CrossRef]
- Lakkaboyana, S.K.; Khantong, S.; Asmel, N.K.; Obaidullah, S.; Kumar, V.; Kannan, K.; Venkateswarlu, K.; Yuzir, A.; Yaacob, W.Z.W. Indonesian Kaolin supported nZVI (IK-nZVI) used for the an efficient removal of Pb(II) from aqueous solutions: Kinetics, thermodynamics and mechanism. J. Environ. Chem. Eng. 2021, 9, 106483. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, L.W.; Zhao, X.; Liu, S.; Li, D. Removal of Pb(II), Cd(II) and Hg(II) from Aqueous Solution by Mercapto-modified Coal Gangue. J. Environ. Manag. 2019, 231, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Liu, Y.; Cheng, L.; Jiang, Z.; Zhang, G.S.; Deng, F.X.; Wang, L.; Han, W.; Zhang, Y. Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms. J. Hazard. Mater. 2021, 403, 123607. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U (VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, W.; Fang, L.; Wang, D. Synthesis of akageneite (beta-FeOOH)/reduced graphene oxide nanocomposites for oxidative decomposition of 2-chlorophenol by Fenton-like reaction. J. Hazard. Mater. 2016, 308, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jeong, S.; Lee, G.; Park, H.; Kim, J. Removal of aqueous-phase Pb(II), Cd(II), As(III), and As(V) by nanoscale zero-valent iron supported on exhausted coffee grounds. Waste Manag. 2019, 92, 49–58. [Google Scholar] [CrossRef]
- Jiao, W.Z.; Song, Y.; Zhang, D.S.; Chang, G.Z.; Fan, H.L.; Liu, Y.Z. Nanoscale zero-valent iron modified with carboxymethyl cellulose in an impinging stream-rotating packed bed for the removal of lead(II). Adv. Powder Technol. 2019, 30, 2251–2261. [Google Scholar] [CrossRef]
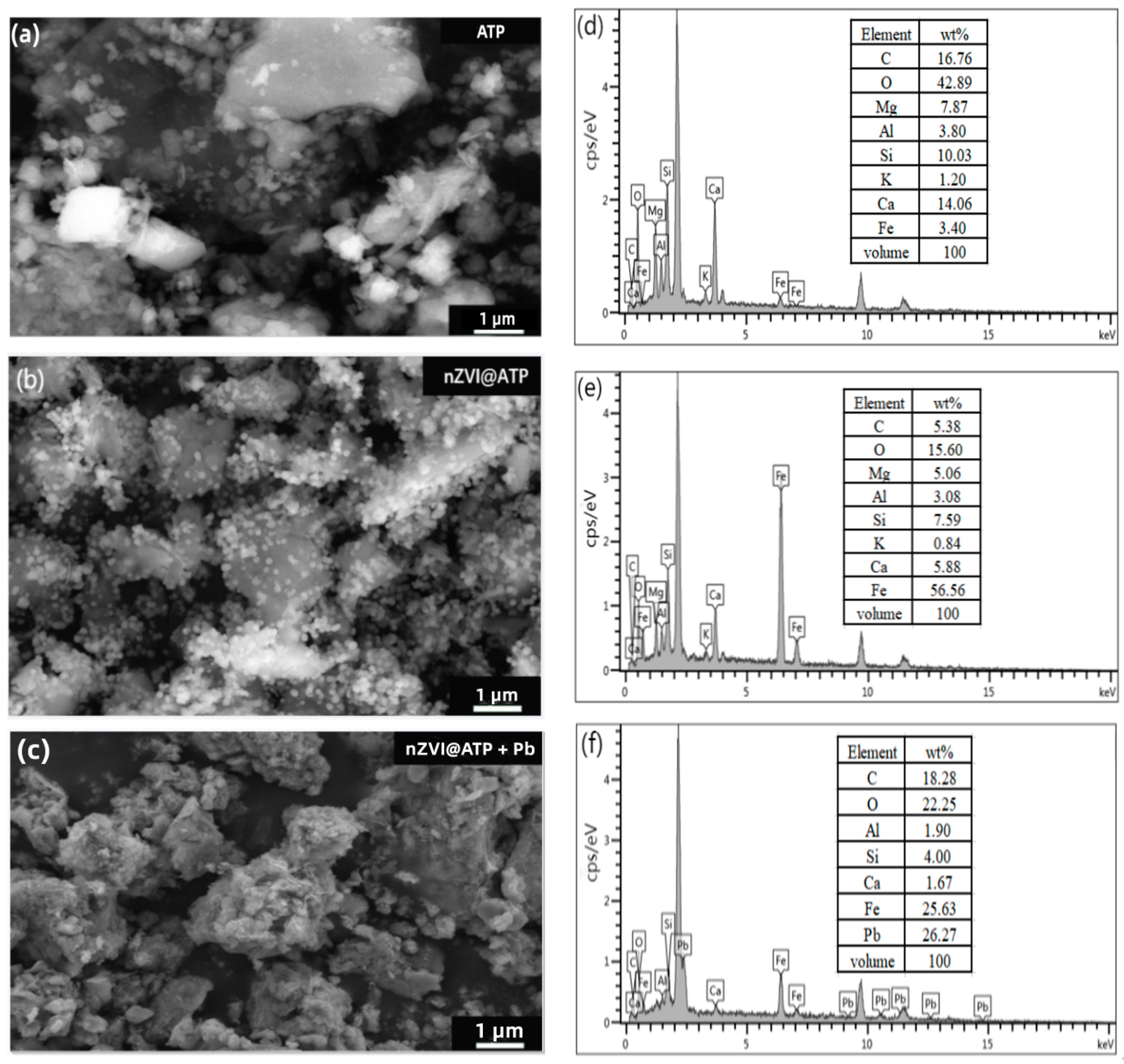

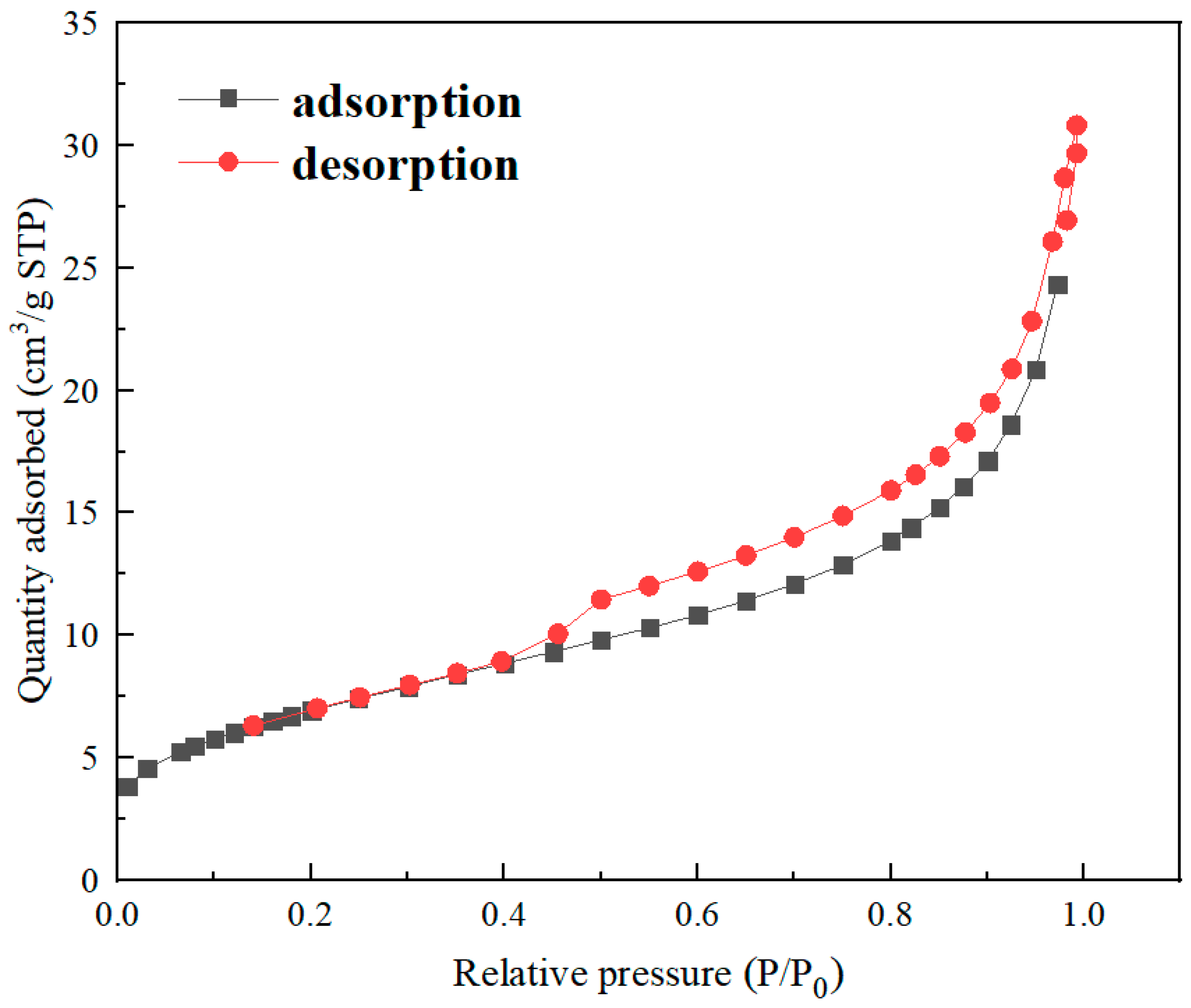


| Sample | nZVI@ATP |
|---|---|
| BET Surface area (m2/g) | 25.413 |
| BJH Adsorption cumulative surface area of pores (m2/g) | 19.993 |
| Single point adsorption total pore volume of pores (cm3/g) | 4.593 × 10−2 |
| BJH Adsorption cumulative volume of pores (cm3/g) | 4.391 × 10−2 |
| Adsorption average pore width (4V/A by BET) (nm) | 7.229 |
| Sample | Carrier | C0 (mg/L) | T (K) | pH | Dosage (g/L) | Qt (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Zeolite and nanoscale zero-valent iron | Zeolite | 100 | 308 | 4 | 1 | 96.2 | [29] |
| Kaolin supported nZVI | Kaolin | 500 | 303.15 | * | 5 | 440.5 | [30] |
| nZVI-HCS | Hydrophilic corn stalk | 50 | 298 | 7 | 0.25 | 195.1 | [31] |
| Montmorillonite-supported nZVI | Montmorillonite | 50 | 298 | 5 | 5 | 10.65 | [32] |
| nZVI@ATP | ATP | 700 | 298 | 5 | 1 | 578.77 | This work |
| C0 | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| qm1 | k1 | R12 | qm2 | k2 | R22 | |
| 700 mg/L | 554.0631 | 3.3173 | 0.7701 | 578.7727 | 0.0098 | 0.9614 |
| C0 | kd1 | E1 | R2 | kd2 | E2 | R2 |
|---|---|---|---|---|---|---|
| 700 mg/L | 124.4606 | 324.7125 | 0.9356 | 7.8813 | 546.9407 | 0.9761 |
| T/K | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | R2 | A | Kt | R2 | ||
| 298 | 618.1546 | 0.4935 | 0.8909 | 486.6824 | 0.0440 | 0.9730 | 25.8522 | 1.0512 × 108 | 0.9759 |
| 308 | 747.8679 | 2.0096 | 0.8456 | 561.5242 | 0.0680 | 0.9842 | 46.1529 | 1.6551 × 105 | 0.9887 |
| 318 | 777.6810 | 24.2099 | 0.7725 | 653.3360 | 0.0548 | 0.9710 | 38.0106 | 3.4127 × 107 | 0.9678 |
| Kd | C0 (mg/L) | ΔHo (kJ/mol) | ΔSo (J/(mol·K)) | ΔGo (kJ/mol) | ||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||||
| Kd = qe/Ce (mL/g) | 550 | 204.876 | 717.688 | −8.995 | −16.172 | −23.349 |
| 600 | 230.271 | 795.176 | −6.692 | −14.643 | −22.595 | |
| 650 | 206.058 | 709.607 | −5.405 | −12.501 | −19.597 | |
| 700 | 127.144 | 441.172 | −4.325 | −8.737 | −13.149 | |
| 750 | 116.786 | 404.169 | −3.657 | −7.698 | −11.740 | |
| 800 | 91.081 | 315.223 | −2.856 | −6.008 | −9.160 | |
| 850 | 87.577 | 302.014 | −2.423 | −5.443 | −8.463 | |
| 900 | 70.817 | 244.507 | −2.046 | −4.491 | −6.936 | |
| 950 | 57.215 | 197.816 | −1.734 | −3.712 | −5.691 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Meng, K.; Zhao, W.; Han, T.; Lei, Z.; Ma, G.; Tian, X.; Ren, J. Mechanism-Enhanced Active Attapulgite-Supported Nanoscale Zero-Valent Iron for Efficient Removal of Pb2+ from Aqueous Solution. Nanomaterials 2022, 12, 1591. https://doi.org/10.3390/nano12091591
Dai L, Meng K, Zhao W, Han T, Lei Z, Ma G, Tian X, Ren J. Mechanism-Enhanced Active Attapulgite-Supported Nanoscale Zero-Valent Iron for Efficient Removal of Pb2+ from Aqueous Solution. Nanomaterials. 2022; 12(9):1591. https://doi.org/10.3390/nano12091591
Chicago/Turabian StyleDai, Liang, Kai Meng, Weifan Zhao, Tao Han, Zhenle Lei, Gui Ma, Xia Tian, and Jun Ren. 2022. "Mechanism-Enhanced Active Attapulgite-Supported Nanoscale Zero-Valent Iron for Efficient Removal of Pb2+ from Aqueous Solution" Nanomaterials 12, no. 9: 1591. https://doi.org/10.3390/nano12091591
APA StyleDai, L., Meng, K., Zhao, W., Han, T., Lei, Z., Ma, G., Tian, X., & Ren, J. (2022). Mechanism-Enhanced Active Attapulgite-Supported Nanoscale Zero-Valent Iron for Efficient Removal of Pb2+ from Aqueous Solution. Nanomaterials, 12(9), 1591. https://doi.org/10.3390/nano12091591