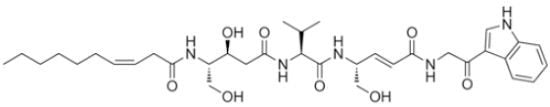
Thalassospiramide G, a New γ-Amino-Acid-Bearing Peptide from the Marine Bacterium Thalassospira sp.
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structural Elucidation
| C/H | δH a | mult (J in Hz) | δC b | |
|---|---|---|---|---|
| 1 | 190.2 | C | ||
| 2 | 114.0 | C | ||
| 3 | 8.44 | s | 133.6 | CH |
| 4 | 136.5 | C | ||
| 5 | 7.50 | d (8.0) | 112.1 | CH |
| 6 | 7.23 | dd (7.5, 7.0) | 122.7 | CH |
| 7 | 7.19 | dd (7.5, 7.0) | 121.6 | CH |
| 8 | 8.16 | d (8.0) | 120.9 | CH |
| 9 | 125.4 | C | ||
| 10 | 4.52 | m | 45.5 | CH2 |
| 10-NH | 8.37 | t (5.5) | ||
| 11 | 164.9 | C | ||
| 12 | 6.13 | d (16.0) | 124.3 | CH |
| 13 | 6.62 | dd (16.0, 6.0) | 140.7 | CH |
| 14 | 4.47 | m | 51.7 | CH |
| 14-NH | 8.18 | d (8.5) | ||
| 15a | 3.46 | m | 62.8 | CH2 |
| 15b | 3.44 | m | ||
| 16 | 171.0 | C | ||
| 17 | 4.25 | dd (9.0, 6.0) | 57.5 | CH |
| 17-NH | 7.92 | Br, s | ||
| 18 | 2.05 | m | 30.2 | CH |
| 19 | 0.86 | m | 19.1 | CH3 |
| 20 | 0.84 | m | 17.6 | CH3 |
| 21 | 171.2 | C | ||
| 22a | 2.35 | dd (15.0, 10.0) | 39.9 | CH2 |
| 22b | 2.17 | dd (15.0, 4.0) | ||
| 23 | 4.12 | m | 65.9 | CH |
| 24 | 3.70 | m | 54.6 | CH |
| 24-NH | 7.54 | d (7.0) | ||
| 25a | 3.46 | m | 60.0 | CH2 |
| 25b | 3.32 | m | ||
| 26 | 170.6 | C | ||
| 27 | 2.97 | m | 33.9 | CH2 |
| 28 | 5.54 | m | 123.7 | CH |
| 29 | 5.46 | m | 131.4 | CH |
| 30 | 2.02 | m | 26.5 | CH2 |
| 31 | 1.31 | m | 28.6 | CH2 |
| 32 | 1.25 | m | 28.2 | CH2 |
| 33 | 1.23 | m | 30.9 | CH2 |
| 34 | 1.26 | m | 21.9 | CH2 |
| 35 | 0.86 | t (6.5) | 13.6 | CH3 |

2.2. Inhibition of NO Production in LPS-Stimulated RAW 264.7 Cells

3. Experimental Section
3.1. General Experimental Procedures
3.2. Bacterial Material, Cultivation and Extraction
3.3. Isolation of Thalassospiramides G, A and D
3.3.1. Thalassospiramide G (1)
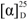 −1.5 (c 0.10, CH3CN); IR (neat) νmax 3420, 1679, 1591 cm−1; 1H and 13C NMR data (see Table 1); UV (CH3CN) λmax(log ε) 204 (4.09), 239 (3.38), 280 (3.08); HR-ESIMS m/z 670.3785 [M + H]+ (calculated for C35H52N5O8 670.3810).
−1.5 (c 0.10, CH3CN); IR (neat) νmax 3420, 1679, 1591 cm−1; 1H and 13C NMR data (see Table 1); UV (CH3CN) λmax(log ε) 204 (4.09), 239 (3.38), 280 (3.08); HR-ESIMS m/z 670.3785 [M + H]+ (calculated for C35H52N5O8 670.3810).3.3.2. Thalassospiramide D (3)
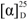 −40.5 (c 0.10, CH3CN); IR (neat) νmax 3395, 2925, 1801, 1662, 1599 cm−1; 1H and 13C NMR data (see Table S1); UV (CH3CN) λmax(log ε) 224 (4.19), 278 (3.26); HR-ESIMS m/z 832.4825 [M + H]+ (calculated for C46H66N5O9 832.4855).
−40.5 (c 0.10, CH3CN); IR (neat) νmax 3395, 2925, 1801, 1662, 1599 cm−1; 1H and 13C NMR data (see Table S1); UV (CH3CN) λmax(log ε) 224 (4.19), 278 (3.26); HR-ESIMS m/z 832.4825 [M + H]+ (calculated for C46H66N5O9 832.4855).3.4. Acid Hydrolysis and Advanced Marfey Analysis
3.5. Bioassay
4. Conclusion
Acknowledgments
References
- Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef]
- Fenical, W. Chemical studies of marine bacteria: Developing a new resource. Chem. Rev. 1993, 93, 1673–1683. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar]
- Gulder, T.A.M.; Moore, B.S. Chasing the treasures of the sea—Bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260. [Google Scholar] [CrossRef]
- Murphy, B.T.; Jensen, P.R.; Fenical, W. The Chemistry of Marine Bacteria. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, O., Eds.; Springer Science: New York, NY, USA, 2012; Volume 1, pp. 153–189. [Google Scholar]
- Rungprom, W.; Siwu, E.R.O.; Lambert, L.K.; Dechsakulwatana, C.; Barden, M.C.; Kokpol, U.; Blanchfield, J.T.; Kita, M.; Garson, M.J. Cyclic tetrapeptides from marine bacteria associated with the seaweed Diginea sp. and the sponge Halisarca ectofibrosa. Tetrahedron 2008, 64, 3147–3152. [Google Scholar]
- Uzair, B.; Ahmed, N.; Ahmad, V.U.; Mohammad, F.V.; Edwards, D.H. The isolation, purification and biological activity of a novel antibacterial compound produced by Pseudomonas stutzeri. FEMS Microbiol. Lett. 2008, 279, 243–250. [Google Scholar] [CrossRef]
- Homann, V.V.; Sandy, M.; Tincu, J.A.; Templeton, A.S.; Tebo, B.M.; Butler, A. Loihichelins A–F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J. Nat. Prod. 2009, 72, 884–888. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.; McAtee, J.; Hemscheidt, T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Sandy, M.; Han, A.; Blunt, J.; Munro, M.; Haygood, M.; Butler, A. Vanchrobactin and anguibactin siderophores produced by Vibrio sp. DS40M4. J. Nat. Prod. 2010, 73, 1038–1043. [Google Scholar] [CrossRef]
- Al-Zereini, W.; Yao, C.B.F.F.; Laatsch, H.; Anke, H. Aqabamycins A–G: Novel nitro maleimides from a marine Vibrio species. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2010, 63, 297–301. [Google Scholar] [CrossRef]
- Yao, C.B.F.F.; Al-Zereini, W.; Fotso, S.; Anke, H.; Laatsch, H. Aqabamycins A–G: Novel nitro maleimides from a marine Vibrio species: II. Structure elucidation. J. Antibiot. 2010, 63, 303–308. [Google Scholar] [CrossRef]
- Cañedo, L.M.; De la Fuente, J.A.; Gesto, C.; Ferreiro, M.J.; Jiménez, C.; Riguera, R. Agrochelin, a new cytotoxic alkaloid from the marine bacteria Agrobacterium sp. Tetrahedron Lett. 1999, 40, 6841–6844. [Google Scholar]
- Takaishi, S.; Tuchiya, N.; Sato, A.; Negishi, T.; Takamatsu, Y.; Matsushita, Y.; Watanabe, T.; Iijima, Y.; Haruyama, H.; Kinoshita, T.; et al. B-90063, a novel endothelin converting enzyme inhibitor isolated from a new marine bacterium, Blastobacter sp. SANK 71894. J. Antibiot. 1998, 51, 805–815. [Google Scholar] [CrossRef]
- Oh, D.-C.; Strangman, W.K.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett. 2007, 9, 1525–1528. [Google Scholar] [CrossRef]
- Ross, A.C.; Xu, Y.; Lu, L.; Kersten, R.D.; Shao, Z.; Al-Suwailem, A.M.; Dorrestein, P.C.; Qian, P.-Y.; Moore, B.S. Biosynthetic multitasking facilitates thalassospiramide structural diversity in marine bacteria. J. Am. Chem. Soc. 2013, 135, 1155–1162. [Google Scholar]
- Hobbs, A.J.; Higgs, A.; Moncada, S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 191–220. [Google Scholar] [CrossRef]
- Preciado, A.; Williams, P.G. A simple microscale method for determining the relative stereochemistry of statine units. J. Org. Chem. 2008, 73, 9228–9234. [Google Scholar] [CrossRef]
- Oh, D.-C.; Poulsen, M.; Currie, C.R.; Clardy, J. Dentigerumycin: A bacterial mediator of an ant-fungus symbiosis. Nat. Chem. Biol. 2009, 5, 391–393. [Google Scholar] [CrossRef]
- Liang, Z.; Sorribas, A.; Sulzmaier, F.J.; Jiménez, J.I.; Wang, X.; Sauvage, T.; Yoshida, W.Y.; Wang, G.; Ramos, J.W.; Williams, P.G. Stictamides A–C, MMP12 inhibitors containing 4-amino-3-hydroxy-5phenylpentanoic acid subunits. J. Org. Chem. 2011, 76, 3635–3643. [Google Scholar]
- Guella, G.; Mancini, I.; N’Diaye, I.; Pietra, F. Almazole C, a new indole alkaloid bearing an unusually 2,5-disubstituted oxazole moiety, and its putative biogenic peptidic precursor, from a Senegalese Delesseriacean seaweed. Helv. Chem. Acta 1994, 77, 1999–2006. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Um, S.; Pyee, Y.; Kim, E.-H.; Lee, S.K.; Shin, J.; Oh, D.-C. Thalassospiramide G, a New γ-Amino-Acid-Bearing Peptide from the Marine Bacterium Thalassospira sp. Mar. Drugs 2013, 11, 611-622. https://doi.org/10.3390/md11030611
Um S, Pyee Y, Kim E-H, Lee SK, Shin J, Oh D-C. Thalassospiramide G, a New γ-Amino-Acid-Bearing Peptide from the Marine Bacterium Thalassospira sp. Marine Drugs. 2013; 11(3):611-622. https://doi.org/10.3390/md11030611
Chicago/Turabian StyleUm, Soohyun, Yuna Pyee, Eun-Hee Kim, Sang Kook Lee, Jongheon Shin, and Dong-Chan Oh. 2013. "Thalassospiramide G, a New γ-Amino-Acid-Bearing Peptide from the Marine Bacterium Thalassospira sp." Marine Drugs 11, no. 3: 611-622. https://doi.org/10.3390/md11030611
APA StyleUm, S., Pyee, Y., Kim, E.-H., Lee, S. K., Shin, J., & Oh, D.-C. (2013). Thalassospiramide G, a New γ-Amino-Acid-Bearing Peptide from the Marine Bacterium Thalassospira sp. Marine Drugs, 11(3), 611-622. https://doi.org/10.3390/md11030611