Okadaic Acid: A Tool to Study the Hippo Pathway
Abstract
:1. Introduction
2. The Mammalian Hippo Pathway
 and
and  panels and ovals represent membrane proteins and membrane-associated proteins of the upstream regulators, respectively.
panels and ovals represent membrane proteins and membrane-associated proteins of the upstream regulators, respectively.  and
and  panels and ovals show core components that include serine/threonine kinases and transcriptional co-activators that are phosphorylated and negatively regulated by kinases. Please refer to text for details.
panels and ovals show core components that include serine/threonine kinases and transcriptional co-activators that are phosphorylated and negatively regulated by kinases. Please refer to text for details.
 and
and  panels and ovals represent membrane proteins and membrane-associated proteins of the upstream regulators, respectively.
panels and ovals represent membrane proteins and membrane-associated proteins of the upstream regulators, respectively.  and
and  panels and ovals show core components that include serine/threonine kinases and transcriptional co-activators that are phosphorylated and negatively regulated by kinases. Please refer to text for details.
panels and ovals show core components that include serine/threonine kinases and transcriptional co-activators that are phosphorylated and negatively regulated by kinases. Please refer to text for details.
3. Regulation of MST1/2 Kinase Activities by Okadaic Acids
 ,
,  and
and  ovals show the upstream regulators, the core components, and the transcriptional co-activators as in Figure 1.
ovals show the upstream regulators, the core components, and the transcriptional co-activators as in Figure 1.  arrows represent the dephosphorylation of various proteins, which are blocked by okadaic acid. Other components of the Hippo pathway such as Mob1 and LATS kinases can also be targets of okadaic acid.
arrows represent the dephosphorylation of various proteins, which are blocked by okadaic acid. Other components of the Hippo pathway such as Mob1 and LATS kinases can also be targets of okadaic acid.
 ,
, 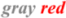 and
and  ovals show the upstream regulators, the core components, and the transcriptional co-activators as in Figure 1.
ovals show the upstream regulators, the core components, and the transcriptional co-activators as in Figure 1.  arrows represent the dephosphorylation of various proteins, which are blocked by okadaic acid. Other components of the Hippo pathway such as Mob1 and LATS kinases can also be targets of okadaic acid.
arrows represent the dephosphorylation of various proteins, which are blocked by okadaic acid. Other components of the Hippo pathway such as Mob1 and LATS kinases can also be targets of okadaic acid.
4. TAZ and YAP as Substrates of PP1 and PP2A
5. Other Candidate Substrates of PP1 and PP2A in the Hippo Pathway
6. Conclusions
Conflict of Interest
References
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Bao, Y.; Hata, Y.; Ikeda, M.; Withanage, K. Mammalian Hippo pathway: From development to cancer and beyond. J. Biochem. 2011, 149, 361–379. [Google Scholar] [CrossRef]
- Ramos, A.; Camargo, F.D. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012, 22, 339–346. [Google Scholar] [CrossRef]
- Varelas, X.; Wrana, J.L. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol. 2012, 22, 88–96. [Google Scholar] [CrossRef]
- Taylor, L.K.; Wang, H.-C.R.; Erikson, R.L. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc. Natl. Acad. Sci. USA 1996, 93, 10099–10104. [Google Scholar] [CrossRef]
- Hong, W.; Guan, K.L. The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 2012, 23, 785–793. [Google Scholar] [CrossRef]
- Bao, Y.; Nakagawa, K.; Yang, Z.; Ikeda, M.; Withanage, W.; Ishigami-Yuasa, M.; Okuno, Y.; Hata, S.; Nishina, H.; Hata, Y. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J. Biochem. 2011, 150, 199–208. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Miller, E.; Yang, J.; DeRan, M.; Wu, C.; Su, A.I.; Bonamy, G.M.; Liu, J.; Peters, E.C.; Wu, X. Identification of serum-derived sphigosine-1-phosphate as a small molecule regulator YAP. Chem. Biol. 2012, 19, 955–962. [Google Scholar] [CrossRef]
- Ling, P.; Lu, T.-J.; Yuan, C.-J.; Lai, M.-D. Biosignaling of mammalian Ste20-related kinases. Cell Signal. 2008, 20, 1237–1247. [Google Scholar] [CrossRef]
- Delpire, E. The mammalian family of sterile 20p-like protein kinases. Pflugers Arch. 2009, 458, 953–967. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. The DeMSTification of mammalian Ste20 kinases. Curr. Biol. 2009, 19, R421–R425. [Google Scholar] [CrossRef]
- O’Neill, E.; Rushworth, L.; Baccarini, M.; Kolch, W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 2004, 306, 2267–2270. [Google Scholar] [CrossRef]
- Kilili, G.K.; Kyrias, J.M. Mammalian Ste20-like kinase (Mst2) indirectly supports Raf-1/ERK pathway activity via maintenance of protein phosphatase-2A catalytic subunit levels and consequent suppression of inhibitory Raf-1 phosphorylation. J. Biol. Chem. 2010, 285, 15076–15087. [Google Scholar] [CrossRef]
- Ribeiro, P.S.; Josue, F.; Wepf, A.; Wehr, M.C.; Rinner, O.; Kelly, G.; Tapon, N.; Gstaiger, M. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 2010, 39, 521–534. [Google Scholar] [CrossRef]
- Matallanas, D.; Romano, D.; Yee, K.; Meissl, K.; Kucerova, L.; Piazzolla, D.; Baccarini, M.; Vass, J.K.; Kolch, W.; O’Neill, E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol. Cell 2007, 27, 962–975. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, X.; Pfeifer, G.P. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J. Biol. Chem. 2011, 286, 6253–6261. [Google Scholar] [CrossRef]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummlkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lv, X.; Li, T.; Xu, Y.; Zhou, X.; Zhao, S.; Xiong, Y.; Lei, Q.-Y.; Guan, K.-L. PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J. Biol. Chem. 2011, 286, 5558–5566. [Google Scholar]
- Wang, P.; Bai, Y.; Song, B.; Wang, Y.; Liu, D.; Lai, Y.; Bi, X.; Yuan, Z. PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS One 2011, 6, e24288. [Google Scholar]
- Bao, Y.; Sumita, K.; Kudo, T.; Withanage, K.; Nakagawa, K.; Ikeda, M.; Ohno, K.; Wang, Y.; Hata, Y. Roles of mammalian sterile 20-like kinase 2-dependent phosphorylations of Mps one binder 1B in the activation of nuclear Dbf2-related kinases. Genes Cells 2009, 14, 1369–1381. [Google Scholar] [CrossRef]
- Hergovich, A. MOB control: Reviewing a conserved family of kinase regulators. Cell Signal. 2011, 23, 1433–1440. [Google Scholar] [CrossRef]
- Avruch, J.; Zhou, D.; Fitamant, J.; Bardeesy, N.; Mou, F.; Barrufet, L.R. Protein kinases of the Hippo pathway: Regulation and substrates. Semin. Cell Dev. Biol. 2012, 23, 770–784. [Google Scholar] [CrossRef]
- Moreno, C.S.; Lane, W.S.; Pallas, D.C. A mammalian homolog of yeast MOB1 is both a member and a putative substrate of striatin family-protein phosphatase 2A complexes. J. Biol. Chem. 2001, 276, 24253–24260. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Y.; Ji, M.; Volle, D.J.; Lewis, R.E.; Tsai, M.-Y.; Dong, J. KIBRA protein phosphorylation is regulated by mitotic kinase Aurora and protein phosphatase 1. J. Biol. Chem. 2011, 286, 36304–36315. [Google Scholar]
- Lee, K.-K.; Yonehara, S. Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST). J. Biol. Chem. 2002, 27, 12351–12358. [Google Scholar]
- Praskova, M.; Khoklatchev, A.; Ortiz-Vega, S.; Avruch, J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 2004, 381, 453–462. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hata, Y.; Timalsina, S.; Maimaiti, S. Okadaic Acid: A Tool to Study the Hippo Pathway. Mar. Drugs 2013, 11, 896-902. https://doi.org/10.3390/md11030896
Hata Y, Timalsina S, Maimaiti S. Okadaic Acid: A Tool to Study the Hippo Pathway. Marine Drugs. 2013; 11(3):896-902. https://doi.org/10.3390/md11030896
Chicago/Turabian StyleHata, Yutaka, Shikshya Timalsina, and Sainawaer Maimaiti. 2013. "Okadaic Acid: A Tool to Study the Hippo Pathway" Marine Drugs 11, no. 3: 896-902. https://doi.org/10.3390/md11030896
APA StyleHata, Y., Timalsina, S., & Maimaiti, S. (2013). Okadaic Acid: A Tool to Study the Hippo Pathway. Marine Drugs, 11(3), 896-902. https://doi.org/10.3390/md11030896



