1. Introduction
The surface of protozoan parasites, such as those belonging to the parasite genus
Leishmania, is responsible for regulating interactions with the extracellular environment and is involved in the absorption of nutrients and signaling pathways. According to Azevedo-Pereira
et al. [
1], there is evidence that heparin-binding proteins (HBPs) present on the surface of
Leishmania spp
. may play important roles in the life cycle of parasites and in defining the success of parasite attachment to and invasion of tissues of the mammalian and invertebrate hosts. In addition, there is evidence that HBPs can promote internalization and signaling in the host cells during the infection time course. More recently, de Castro Côrtes
et al. [
2] isolated metallo-protease protein fractions from the flagella or membrane promastigotes of
L. (V) braziliensis that were capable of forming stable complexes with glycosaminoglycans (heparin-like activity). Additionally, de Castro Côrtes
et al. [
3] showed that these HBPs modulate the activity of signaling in the cellular environment and play specific roles in the host-parasite interactions of
Leishmania (V.) braziliensis.
Although heparin is not found on the surface of host cells, this glycosaminoglycan (GAG) has been commonly used as a tool for studying pathogen-host cell interactions. It was previously demonstrated that amastigotes of
L. (L.) amazonensis and
Leishmanai (L.) major have a greater ability to bind to heparin than promastigotes of these same species. In addition, GAGs, including heparin, can induce the proliferation of
L. (L.) major in the gut of the insect vector, increasing the load of experimentally infected insects. There is evidence that the HBPs present on the surface of
Leishmania spp. can play an important role in the life cycle of parasites, defining the success of parasite binding and invasion of the host tissues of mammals and invertebrates. In the parasite species in which these proteins were identified, the HBPs act as adhesion proteins and can promote internalization and signaling in host cells [
2].
Marine macroalgae have high concentrations of sulfated polysaccharides (SP), heterogeneous and complex macromolecules with significant importance to the physiology of algae. These compounds have been explored in the biomedical field, indicating the therapeutic potential of sulfated polysaccharides [
4]. In this respect, extracts produced from green, brown, and red algae have already demonstrated significant activity against fungi, bacteria, viruses and protozoa [
5,
6]. However, until now, few works have demonstrated the anti-leishmanial activity of seaweed extracts. Recently, results have shown that the selective index of extracts is very high in different experimental models, suggesting low toxicity of algae extracts to mammalian cells, which in turn can be explored to develop new candidate drugs against diseases and to examine different pathological conditions. In this study, we evaluated the effects of four highly purified sulfated polysaccharides from
Solieria filiformis (Sf),
Botryocladia occidentalis (Bo),
Caulerpa racemosa (Cr) and
Gracilaria caudate (Gc) on the growth of
Leishmania (L.) amazonensis promastigote, as well as their cytotoxic effects.
2. Results and Discussion
The sulfated polysaccharides used in this study were extensively purified from the crude extracts of
Solieria filiformis (Sf),
Botryocladia occidentalis (Bo),
Caulerpa racemosa (Cr) and
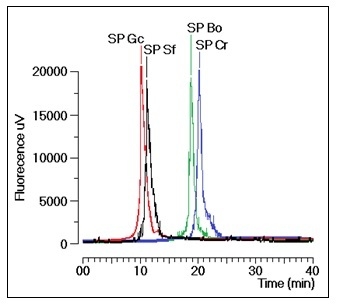
Gracilaria caudate (Gc) by Diethylamionoethyl (DEAE) ion exchange chromatography. These fractions were then subjected to a new chromatography process using a TSKgel G3000SW column with a molecular size exclusion stationary phase previously equilibrated with phosphate buffer. The size exclusion chromatographic analyses of the sulfated polysaccharides (SP) fractions from the DEAE column of Gc, Sf, Bo and Cr showed several fractions, including one main peak that corresponded to approximately 35% of the whole fraction (
Figure 1). The molecular mass of Gc, Sf, Bo and Cr were estimated to be greater than 200 kDa, 30 kDa and 25 kDa, respectively, according to the molecular mass markers: blue dextran (2000 kDa); β-amylase (225 kDa); and bovine serum albumin (BSA, Sigma, 66 kDa), which forms a natural dimer of 120 kDa; ovalbumin (OVA, 43 kDa); carbonic anhydrase (CA, 29 kDa); and ribonuclease (RA, 14 kDa).
Figure 1.
Chromatographic profile of the size exclusion fractionation of sulfated polysaccharides in TSKgel G3000SW silica-based GFC columns. Samples of 1 mg/mL were dissolved in phosphate buffer and centrifuged at 4500× g for 5 min, and the supernatant was applied to the column. The flow rate was maintained at 1 mL/min, and the eluent was monitored by fluorescence. The purified fractions of the sulfated polysaccharides were named SP.
Figure 1.
Chromatographic profile of the size exclusion fractionation of sulfated polysaccharides in TSKgel G3000SW silica-based GFC columns. Samples of 1 mg/mL were dissolved in phosphate buffer and centrifuged at 4500× g for 5 min, and the supernatant was applied to the column. The flow rate was maintained at 1 mL/min, and the eluent was monitored by fluorescence. The purified fractions of the sulfated polysaccharides were named SP.
The SPCr significantly reduced the viability of the promastigotes of
L. (L.) amazonensis in a dose-dependent manner and showed a low EC
50 (34.5 μg/mL) (
Figure 2). Previous studies showed that the crude extracts of cold water
Caulerpa sertularioides inhibited the growth rate of the promastigotes of
Leishmania major and had an EC
50 of 65 μg/mL [
7] (
Table 1). Moreover, the methanol extract of
Caulerpa racemosa increased the leishmanicidal effect on the promastigotes of
L. donovani and was able to eliminate 50% of the promastigotes with a dose of 22.6 μg/mL of extract [
8]. Additionally, the SP
C. racemosa used in this study showed moderate cytotoxic effects in the J774 macrophage cell line (CC
50 = 49.3 μg/mL) (
Figure 3). These results suggest that SPCr may present potential leishmanicidal activity. These results can be extended by the studies of Süzgeç-Selçuk [
8], which have shown that concentrations higher than 90 μg/mL of a crude extract of methanol were required to remove 50% of the L6 cell population. The chemical heterogeneity of this fraction found in the crude extract and the sensibility of cell lineage should result in a higher EC
50 than those found in the present work.
SPBo also significantly reduced the viability of the promastigotes of
L. (L.) amazonensis in a dose-dependent relation and showed a lower EC
50 (58.8 μg/mL) (
Figure 2). Although these results demonstrated that the sulfated polysaccharides showed good activity against the growth of
L. (L.) amazonensis, there are few studies showing other applications of algae of the genus
Botryocladia. There are some studies with
B. leptopoda. One of these studies showed that the crude extract and hexane fraction brought about a marked reduction in the peripheral microfilarial level in the rodent filarial parasites [
9]. Moreover, studies with steroidal alkaloids from
B. leptopoda showed moderate
in vitro anti-leishmanial activity of approximately 60.81 ± 0.41 μg/mL [
10]. Recent studies by Sabina
et al. [
11] showed that ethanol extracts of algae, including
B. leptopoda, also showed significant
in vitro anti-leishmanial activity. The analysis of the results obtained from the
in vitro treatment of
L. (L.) amazonensis promastigotes with SPBo were significantly better than the
in vitro anti-leishmanial activity from secondary metabolites because the molecular weights of the natural compounds are smaller than 1 kDa and the sulfated polysaccharides of
B. occidentalis had a molecular mass of 30 kDa. Studies conducted by Sabina
et al. [
11] identified that the flavone extracted from the red seaweed
Osmundea pinnatifida showed inhibition of viability in tests with
Leishmania. The SP from
B. occidentalis had the second most potent cytotoxic effects on the J774 macrophage cell line (CC
50 = 27.3 μg/mL) (
Figure 3) and the lowest selectivity index (SI = 0.42) (
Table 1).
Figure 2.
Anti-promastigote effects of SP from the seaweeds Solieria filiformis (Sf), Botryocladia occidentalis (Bo), Caulerpa racemosa (Cr), and Gracilaria caudata (Gc) on L. (L.) amazonensis. * p < 0.05 indicates significant anti-promastigote effects compared to untreated promastigote forms.
Figure 2.
Anti-promastigote effects of SP from the seaweeds Solieria filiformis (Sf), Botryocladia occidentalis (Bo), Caulerpa racemosa (Cr), and Gracilaria caudata (Gc) on L. (L.) amazonensis. * p < 0.05 indicates significant anti-promastigote effects compared to untreated promastigote forms.
Table 1.
Determination of the EC50 and CC50 values and selectivity indices of the purified sulfated polysaccharides in comparison with Amphotericin B.
Table 1.
Determination of the EC50 and CC50 values and selectivity indices of the purified sulfated polysaccharides in comparison with Amphotericin B.
| Algae species | EC50 (μg/mL) | CC50 (μg/mL) | Selectivity index |
|---|
| C. racemosa | 34.5 | 49.3 | 1.42 |
| B. occidentalis | 63.7 | 27.3 | 0.42 |
| S. filiformis | 137.4 | 99.8 | 0.72 |
| G. caudate | No activity | 73.2 | Not determined |
| Amphotericin B | 0.05 | ≥90 | - |
Figure 3.
Cytotoxic effects of SP from the seaweeds Sf, Bo, Cr, and Gc on J774 macrophages. * p < 0.05 indicates significant cytotoxic effects compared to untreated J774 cells.
Figure 3.
Cytotoxic effects of SP from the seaweeds Sf, Bo, Cr, and Gc on J774 macrophages. * p < 0.05 indicates significant cytotoxic effects compared to untreated J774 cells.
In contrast, the SP from
Solieria filiformis presented the highest anti-leishmanial values (EC
50 = 137.4 μg/mL) (
Figure 2) and cytotoxic activities (CC
50 = 99.8 μg/mL) (
Figure 3). The selectivity index was 0.72 μg/mL, thus suggesting that the cytotoxicity is responsible for killing the leishmania, rather than being a direct effect on the promastigote forms (
Table 1). The SP from
Gracilaria caudata algae showed no anti-leishmanial effect; however, a CC
50 of 73.2 μg/mL was reached. Studies by Fouladvand
et al. [
7] investigated the IC
50 values for the hot water extracts of
Gracilaria corticata (IC
50 ≤ 38 μg/ mL),
Gracilaria salicornia (IC
50 ≤ 46 μg/ mL) and
Gracilaria salicornia (IC
50 > 105 μg/mL) and the cold water extract of
Gracilaria corticata (IC
50 > 74 μg/mL). Studies on the anti-leishmanial effects of Mediterranean red algae showed that halogenated compounds from these algae have strong leishmanicidal activities [
7].
The seaweed-derived SP tested in this study significantly inhibited the promastigote growth rate
in vitro. Some of the observed IC
50 values were comparable to those of other natural products, such as other organic small molecules isolated from algae sources. This article is the first report of the anti-leishmanial activity of algae SP. Our results also showed that none of the SP assayed here significantly modified the peritoneal macrophage viability (
Figure 4). The difference between the cytotoxicity of the J774 macrophages and macrophages collected from the peritoneum is the resulting heterogeneity in the intracellular survival among the various macrophage-sensitive mutants, which may reflect the relative importance of the individual mutated genes in survival within macrophages [
12]. The
in vitro anti-leishmanial activity of sulfated polysaccharides depends on the molecular size of the sulfated polysaccharides. Our results show that the SP with high molecular weights showed the worst inhibition results. In the case of the sulfated polysaccharides of
Botryocladia occidentalis and
Caulerpa racemosa, the difference in molecular weight is small; therefore, the chemical composition of both sulfated polysaccharides can determine the best
in vitro anti-leishmanial activity.
Figure 4.
Cytotoxic effects of SP from the seaweeds Sf, Bo, Cr, and Gc on peritoneal macrophages. * p < 0.05 indicates significant cytotoxic effects compared to untreated peritoneal macrophage.
Figure 4.
Cytotoxic effects of SP from the seaweeds Sf, Bo, Cr, and Gc on peritoneal macrophages. * p < 0.05 indicates significant cytotoxic effects compared to untreated peritoneal macrophage.
3. Experimental Section
3.1. Specimen Collection
Solieria filiformis (Sf), Botryocladia occidentalis (Bo), Caulerpa racemosa (Cr) and Gracilaria caudata (Gc) were collected from the coast of Fortaleza, Brazil during the summer. After collection, all samples were washed thoroughly under tap water, packed in plastic bags, transported to the laboratory on dry ice, washed with Milli Q water, and stored at −80 °C until the extracts were prepared. The fraction of SP from seaweed was supplied by Prof. Dr. Wladimir R. L. Farias (group of Department de Engenharia de Pesca/Biologia Molecular e Biologia Marinha, UFC, Fortaleza, Ceará, Brazil).
3.2. Fractionation of the SP from Algae
All of the dry seaweed (5 g) was triturated and hydrated in 250 mL of 0.1 M sodium acetate, 5 mM cysteine and 5 mM EDTA, pH 5.0. Immediately afterward, 17 mL of crude papain solution (30 mg/mL) was added, and the mixture was incubated in a water bath at 60 °C for 24 h. The material was then filtered and centrifuged (14,000 rpm, 30 min, 4 °C). After this step, 16 mL of cetylpiridinium chloride (CPC) was added to provide a final concentration of 10%. The solution was incubated at room temperature for 24 h to allow the polysaccharides to precipitate. The SPs were washed with 500 mL CPC, dissolved in 174 mL of 2 M NaCl:ethanol (100:15, v:v) in a water bath at 60 °C, and precipitated again by the addition of 305 mL of absolute ethanol for 24 h at 4 °C. The material was centrifuged again and washed successively with 500 mL of absolute ethanol and 300 mL of 80% ethanol. The SPs were dried in an oven at 60 °C for 24 h to obtain the SP-rich fraction. The crude extracts were purified on a DEAE-cellulose resin equilibrated with 0.1 M sodium acetate, 5 mM cysteine, and 5 mM EDTA, pH 5.0. The polysaccharides adsorbed onto the ion exchange resin were eluted with 1.5 M NaCl using gel equilibration buffer. The quantification of SP was carried out through a metachromatic reaction using a spectrophotometer at λmax 525 nm. The high-performance liquid chromatography instrumentation was from Jasco (Jasco Analytical Instruments; 28600 Mary’s Court Easton, MD 21601, USA): Model PU-2080 pump; Chronav system controller; Rheodyne injector equipped with a 20-μL loop; FP-2020 fluorescence detector, MD-2015/2018 diode array arrangement detector and an ELC-2041 evaporative light scattering detector. The TSK G3000SWXL SEC column (0.7 × 300 mm) was equilibrated with phosphate buffer (0.05 M, pH 7.5) for 30 min at a flow rate of 1 mL/min before sample addition. Aliquots of 200 μL of sulfated polysaccharide were loaded on the column. The elution was performed at a flow rate of 1 mL/min and was monitored by UV-Vis detection at λmax 280 nm. For this study, we used the protein markers blue dextran (BD, 2000 kDa); β-amylase (BA, 225 kDa); bovine serum albumin (BSA, Sigma, 66 kDa), which forms a natural dimer of 120 kDa, ovalbumin (OVA, 43 kDa); carbonic anhydrase (CA, 29 kDa); and ribonuclease (RA, 14 kDa) to estimate the molecular weight of the various SP. All samples were analyzed under the same buffer, flow rate and column chromatographic conditions.
3.3. Determination of Anti-Leishmanial Activity
L. (L.) amazonensis (MHOM/BR/73/M2269 strain) parasite was classified on the basis of characteristic monoclonal antibodies and isoenzymes at the Evandro Chagas Institute, Belém, PA, Brazil. This strain was maintained in the footpad of BALB/c mice to keep their infectivity. The parasites were isolated, cultured, and maintained at 25 °C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 0.25 mM HEPES, gentamicin (10 μg/mL), and penicillin (100 IU/mL) (R10 medium). The anti-leishmanial assays were performed following the protocol described by Passero
et al. [
13]. Briefly, promastigotes in the stationary phase of growth were washed three times with phosphate buffer solution (PBS) and suspended in RPMI 1640 at a concentration of 107 promastigote/mL in a 96-well plate. The SP were solubilized in PBS and sterilized by filtration through a 0.22 μm membrane before being added to the culture. The SPs were individually added to the culture of
L. (L.) amazonensis in a range of 20 to 160 μg/mL. After 24 h of incubation at 25 °C, the viability of the promastigote forms were analyzed through the conversion of 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium bromide (MTT) to formazan. Briefly, the plates were washed three times while shaking at 3000 rpm for 10 min at 4 °C, and 40 μL of R10 medium plus 10 μL of MTT (5 mg/mL) were added to each well. After 4 h, 50 μL of 10% sodium dodecyl sulfate was added to each well. The plates were further incubated for 18 h. Next, the plates were read in an ELISA reader at λ
max 595 nm, and the 50% effective concentration (IC
50) of each SP was calculated with the aid of Origin 5.0 statistical software (Microcal Software, Northampton, MA, USA). Each assay was conducted in triplicate. Amphotericin B was used as the standard drug.
3.4. Macrophage Cytotoxicity
The cytotoxicity of the SPs was assayed against the mouse macrophage cell line J774 and BALB/c-peritoneal macrophages [
14] using a colorimetric reaction based on the MTT assay. Briefly, both cell populations were plated at 106 cell/mL in a 96-well plate. The SPs (at the concentrations described above) were incubated with the cells for 24 h at 35 °C and 5% CO
2. Following the incubation, the plates were washed three times with PBS, and MTT solution was added as described in the determination of anti-leishmanial activity section. The cytotoxic concentration 50% inhibitory concentration (cytotoxic concentration; CC
50) of each SP was calculated with the aid of Origin 5.0 statistical software (Microcal Software, Northampton, MA, USA). Each assay was conducted in triplicate.
3.5. Selectivity Index Determination
To determine the selectivity index (SI), the ratio of the CC
50 value of the cytotoxic activity to the EC
50 value of the antiprotozoal activity was calculated. When the SI value is >10, that compound presents antiprotozoal activity that is higher rather than its cytotoxicity [
15].
3.6. Lethality Assays
Each SP was separately injected into the tail vein of ten mice in serial doses diluted in 100 μL saline (0.3–40 μg/100 g of body weight). The survival times of the animals were recorded for 24 h.
3.7. Statistical Analysis
The results are expressed as the means ± standard deviation. The data were analyzed using Student’s t test. The level of significance was set at p < 0.05.