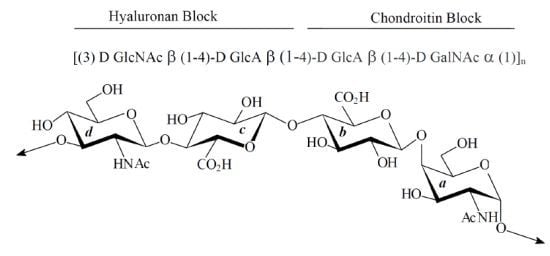
Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues
Abstract
:1. Introduction
2. Results
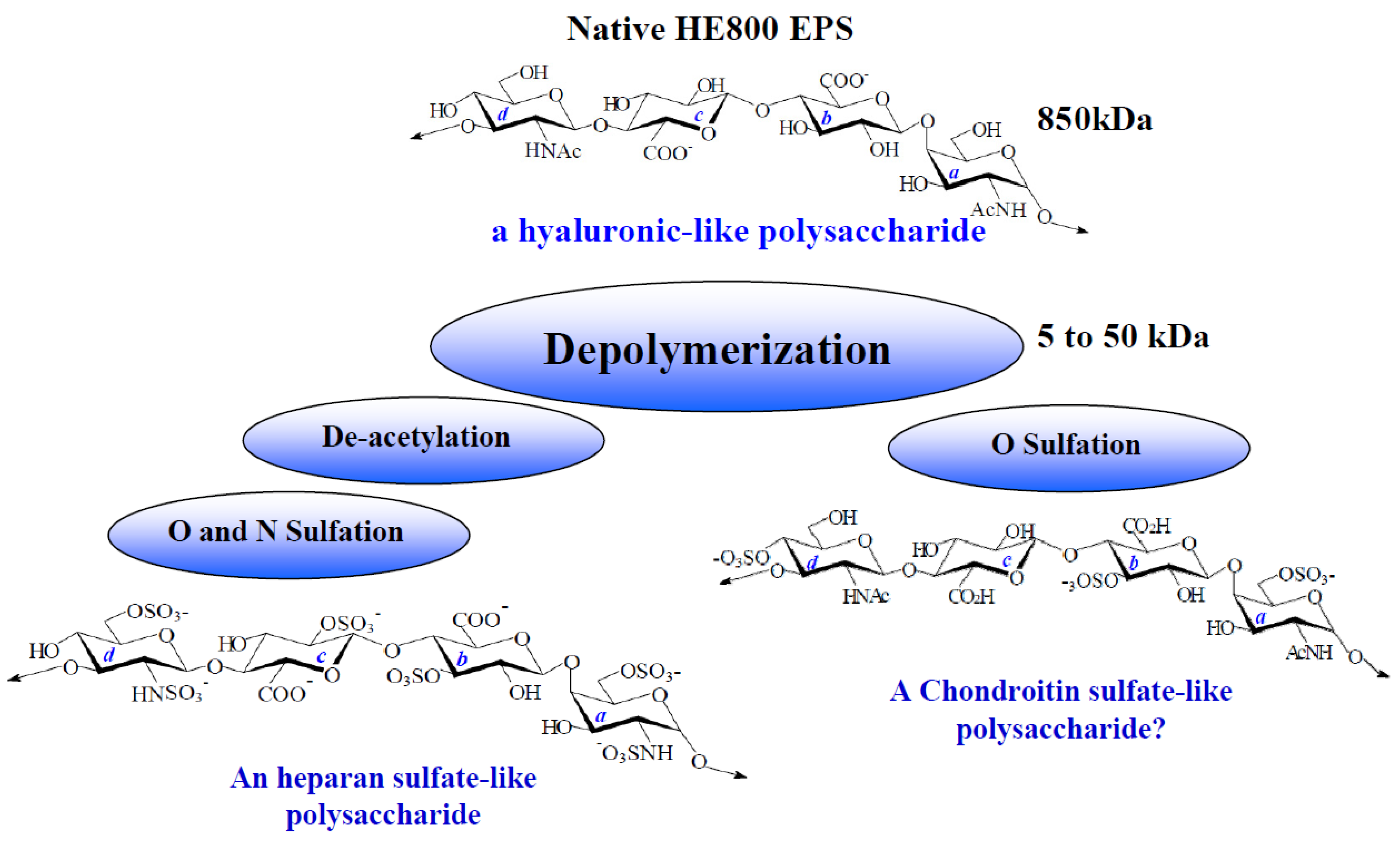
2.1. Characterization of Native HE800 EPS and Its Sulfated Low-Molecular Weight Derivative
| Native HE800 EPS | HE800 DROS | |
|---|---|---|
| Neutral sugars a (%) | 0 | 0 |
| Hexosamines a (%) | 38 | 20 |
| Uronic acids a (%) | 39 | 20 |
| SO3Na b (%) | 0 | 34 |
| Mw c (g/mol) | 850,000 | 22,000 |
| Ip | Not determined | 1.4 |

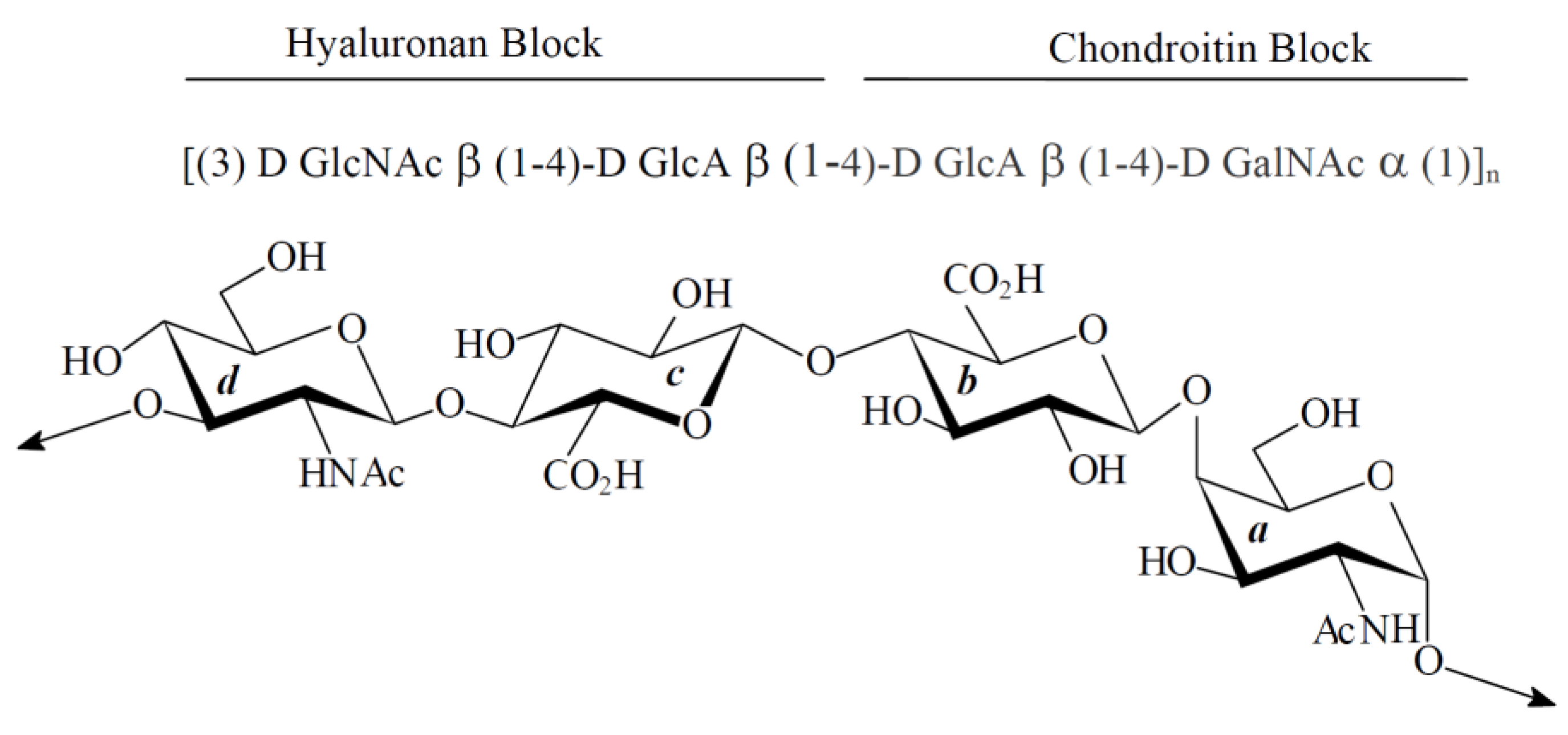
2.2. Effect of HE800 EPS on Collagen Film Structuring
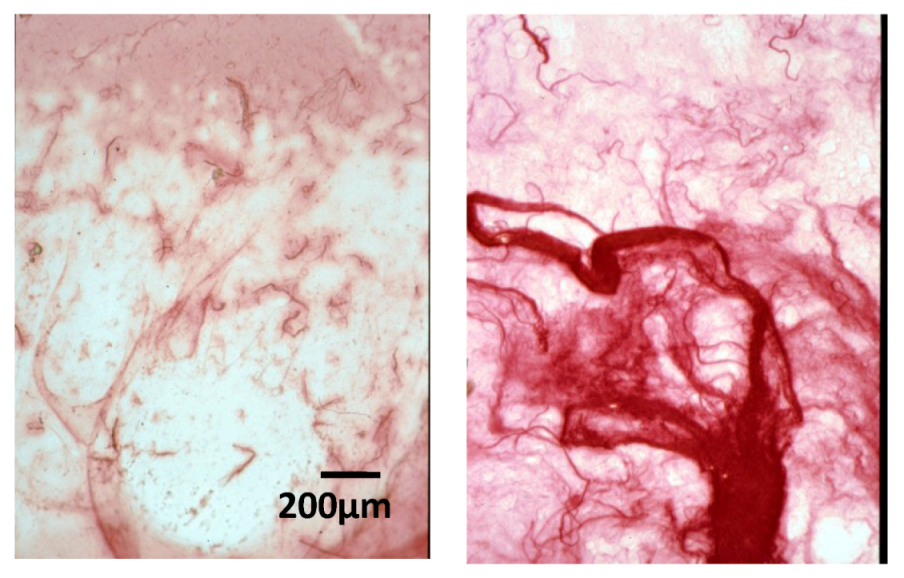
2.3. Effect of Native HE800 EPS on Collagen Matrix Structuring in Reconstructed Connective Tissue (RCT)
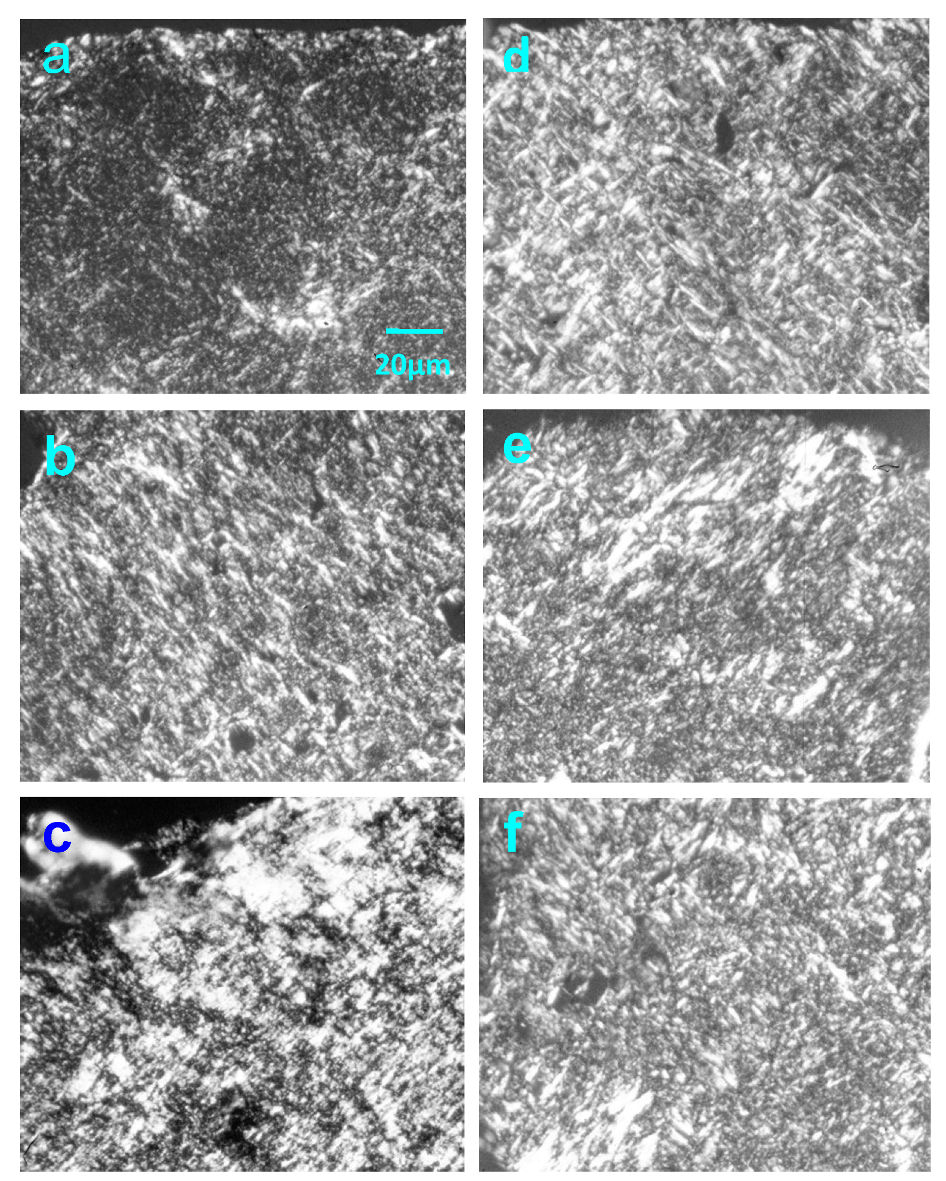
2.4. Effect of Native HE800 EPS on Collagen Fibrillogenesis in Reconstructed Connective Tissue

2.5. Effect of Native HE800 EPS on Fibroblast Densities in Reconstructed Connective Tissues
| Extracellular Matrix | Periphery | |
|---|---|---|
| Control | −2.7% | −22.9% |
| HE800 EPS (300 μg) | +48.9% (**) | −53.6% (**) |
| HE800 EPS (150 μg) | +68.1 (**) | −68.4% (***) |
| HE800 EPS (75 μg) | +54.5% (**) | −75.2% (**) |
2.6. Effect of HE800 DROS Derivative on Matrix Metalloproteinase Secretions
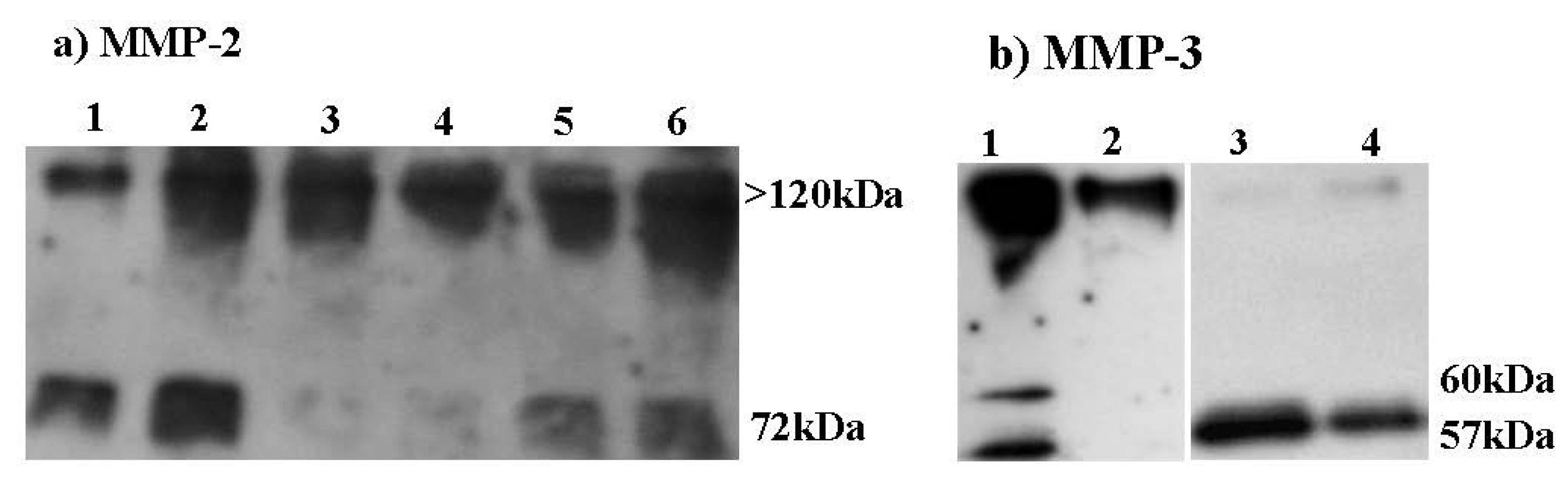
2.7. Effect of HE800 DROS on Dermal Fibroblast Proliferation

3. Discussion
4. Experimental Section
4.1. HE800 EPS
4.2. Human Dermal Fibroblasts
4.3. Collagen and Collagen-HE800 EPS Films
4.4. Reconstructed Connective Tissues (RCT)
4.5. RCT Histology and Cell Counting by Image Analysis
4.6. Transmission Electron Microscopy
4.7. MMPs Studies in Bidimensional Fibroblast Cultures
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusion
Acknowledgments
References
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Iozzo, R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef]
- Juhlin, L. Hyaluronan in skin. J. Int. Med. 1997, 242, 61–66. [Google Scholar]
- Yayon, A.; Klagsbrun, M.; Esko, J.D.; Leder, P.; Ornitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar] [CrossRef]
- Yu, W.H.; Woessner, J.F., Jr.; McNeish, J.D.; Stamenkovic, I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodelling. Genes Dev. 2002, 16, 307–323. [Google Scholar] [CrossRef]
- Stringer, S.E. The role of heparan sulphate proteoglycans in angiogenesis. Biochem. Soc. Trans. 2006, 34, 451–453. [Google Scholar] [CrossRef]
- Feige, J.J.; Baird, A. Crinopexy: Extracellular regulation of growth factor action. Kidney Int. Suppl. 1995, 49, S15–S18. [Google Scholar]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar]
- Scott, J.E. Proteoglycan:Collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J. Anat. 1990, 169, 23–35. [Google Scholar]
- Chung, C.Y.; Erickson, H.P. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J. Cell Sci. 1997, 110, 1413–1419. [Google Scholar]
- Wu, W.J.; Vrhovski, B.; Weiss, A.S. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J. Biol. Chem. 1999, 274, 21719–21724. [Google Scholar] [CrossRef]
- McPherson, J.M.; Sawamura, S.J.; Condell, R.A.; Rhee, W.; Wallace, D.G. The effects of heparin on the physicochemical properties of reconstituted collagen. Coll. Relat. Res. 1988, 8, 65–82. [Google Scholar] [CrossRef]
- Buczek-Thomas, J.A.; Chu, C.L.; Rich, C.B.; Stone, P.J.; Foster, J.A.; Nugent, M.A. Heparan sulfate depletion within pulmonary fibroblasts: Implications for elastogenesis and repair. J. Cell Physiol. 2002, 192, 294–303. [Google Scholar] [CrossRef]
- Volpi, N. Inhibition of human leukocyte elastase activity by chondroitin sulfates. Chem. Biol. Interact. 1997, 105, 157–167. [Google Scholar] [CrossRef]
- Hornebeck, W.; Lafuma, C.; Robert, L.; Móczár, M.; Móczár, E. Heparin and its derivatives modulate serine proteinases (SERPS) serine proteinase inhibitor (SERPINS) balance. Physiopathological relevance. Pathol. Res. Pract. 1994, 190, 895–902. [Google Scholar]
- Kenagy, R.D.; Nikkari, S.T.; Welgus, H.G.; Clowes, A.W. Heparin inhibits the induction of three matrix metalloproteinases (stromelysin, 92-kD gelatinase, and collagenase) in primate arterial smooth muscle cells. J. Clin. Invest. 1994, 93, 1987–1993. [Google Scholar] [CrossRef]
- Gogly, B.; Dridi, M.; Hornebeck, W.; Bonnefoix, M.; Godeau, G.; Pellat, B. Effect of heparin on the production of matrix metalloproteinases and tissue inhibitors of metalloproteinases by human dermal fibroblasts. Cell Biol. Int. 1999, 23, 203–209. [Google Scholar] [CrossRef]
- Kawashima, H. Roles of sulfated glycans in lymphocyte homing. Biol. Pharm. Bull. 2006, 29, 2343–2349. [Google Scholar] [CrossRef]
- Taylor, K.R.; Gallo, R.L. Glycosaminoglycans and their proteoglycans: Host-Associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006, 20, 9–22. [Google Scholar] [CrossRef]
- Croce, M.A.; Dyne, K.; Boraldi, F.; Quaglino, D., Jr.; Cetta, G.; Tiozzo, R.; Pasquali Ronchetti, I. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 2001, 33, 326–331. [Google Scholar] [CrossRef]
- Meyer, L.J.; Russell, S.B.; Russell, J.D.; Trupin, J.S.; Egbert, B.M.; Shuster, S.; Stern, R. Reduced hyaluronan in keloid tissue and cultured keloid fibroblasts. J. Invest. Dermatol. 2000, 114, 953–959. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–69. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zanchetta, P.; Helley, D.; Ratiskol, J.; Sinquin, C.; Fischer, A.M.; Guezennec, J. Microbial polysaccharides of marine origin and their potential in human therapeutics. Pathol. Biol. (Paris) 2004, 52, 127–130. [Google Scholar]
- Simon-Colin, C.; Raguénès, G.; Cozien, J.; Guezennec, J.G. Halomonas profundus sp. nov., a new PHA-producing bacterium isolated from a deep-sea hydrothermal vent shrimp. J. Appl. Microbiol. 2008, 104, 1425–1432. [Google Scholar]
- Guezennec, J. From extreme environments to biologically active exopolysaccharides. Commun. Agric. Appl. Biol. Sci. 2003, 68, 227–234. [Google Scholar]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Mar Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Raguénès, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Bacteriol. 1997, 47, 989–995. [Google Scholar] [CrossRef]
- Rougeaux, H.; Kervarec, N.; Pichon, R.; Guezennec, J. Structure of the exopolysaccharide of Vibrio diabolicus isolated from a deep-sea hydrothermal vent. Carbohydr. Res. 1999, 322, 40–45. [Google Scholar] [CrossRef]
- Loaëc, M.; Olier, R.; Guezennec, J. Uptake of lead, cadmium and zinc by a novel bacterial exopolysaccharide. Water Res. 1997, 31, 1171–1179. [Google Scholar] [CrossRef]
- Zanchetta, P.; Lagarde, N.; Guezennec, J. A new bone-healing material: A hyaluronic acid-like bacterial exopolysaccharide. Calcif. Tissue Int. 2003, 72, 74–79. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar]
- Stuart, K.; Panitch, A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers 2008, 89, 841–851. [Google Scholar] [CrossRef]
- Salchert, K.; Oswald, J.; Streller, U.; Grimmer, M.; Herold, N.; Werner, C. Fibrillar collagen assembled in the presence of glycosaminoglycans to constitute bioartificial stem cell niches in vitro. J. Mater. Sci. Mater. Med. 2005, 16, 581–585. [Google Scholar]
- Helary, C.; Foucault-Bertaud, A.; Gaston Godeau, G.; Coulomb, B.; Giraud Guille, M.M. Fibroblast populated dense collagen matrices: Cell migration, cell density and metalloproteinases expression. Biomaterials 2005, 26, 1533–1543. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Yousfi, M.; Fioretti, F.; Godeau, G.; Colliec-Jouault, S.; Ratiskol, J.; Sinquin, C.; Raguenes, G.; Courtois, A.; et al. Sulfated Depolymerized Derivatives of Exopolysaccharides (EPS) from Mesophilic Marine Bacteria, Method for Preparing Same, and Use Thereof in Tissue Regeneration. U.S. Patent 2008/0,131,472, 5 June 2008. [Google Scholar]
- Docherty, R.; Forrester, J.V.; Lackie, J.M.; Gregory, D.W. Glycosaminoglycans facilitate the movement of fibroblasts through three-dimensional collagen matrices. J. Cell Sci. 1989, 92, 263–270. [Google Scholar]
- Hayen, W.; Goebeler, M.; Kumar, S.; Riessen, R.; Nehls, V. Hyaluronan stimulates tumor cell migration by modulating the fibrin fiber architecture. J. Cell Sci. 1999, 112, 2241–2251. [Google Scholar]
- Boraldi, F.; Croce, M.A.; Quaglino, D.; Sammarco, R.; Carnevali, E.; Tiozzo, R.; Pasquali-Ronchetti, I. Cell-Matrix interactions of in vitro human skin fibroblasts upon addition of hyaluronan. Tissue Cell 2003, 35, 37–45. [Google Scholar]
- Kitamura, M.; Maruyama, N.; Mitarai, T.; Nagasawa, R.; Yokoo, T.; Sakai, O. Heparin selectively inhibits gene expression of matrix metalloproteinase transin in cultured mesangial cells. Biochem. Biophys. Res. Commun. 1994, 203, 1333–1338. [Google Scholar] [CrossRef]
- Imada, K.; Oka, H.; Kawasaki, D.; Miura, N.; Sato, T.; Ito, A. Anti-Arthritic action mechanisms of natural chondroitin sulfate in human articular chondrocytes and synovial fibroblasts. Biol. Pharm. Bull. 2010, 33, 410–414. [Google Scholar] [CrossRef]
- Legendre, F.; Baugé, C.; Roche, R.; Saurel, A.S.; Pujol, J.P. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL-1beta-stimulated chondrocytes-study in hypoxic alginate bead cultures. Osteoarthr. Cartil. 2008, 16, 105–114. [Google Scholar] [CrossRef]
- Miralem, T.; Templeton, D.M. Heparin inhibits Ca2+/calmodulin-dependent kinase II activation and c-fos induction in mesangial cells. Biochem. J. 1998, 330, 651–657. [Google Scholar]
- Butler, G.S.; Apte, S.S.; Willenbrock, F.; Murphy, G. Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions. J. Biol. Chem. 1999, 274, 10846–10851. [Google Scholar]
- Morvan, F.O.; Baroukh, B.; Ledoux, D.; Caruelle, J.P.; Barritault, D.; Godeau, G.; Safar, J.L. An engineered biopolymer prevents mucositis induced by 5-fluorouracil in hamsters. Am. J. Pathol. 2004, 164, 739–746. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef]
- Kovensky, J.; Duchaussoy, P.; Bono, F.; Salmivirta, M.; Sizun, P.; Herbert, J.M.; Petitou, M.; Sinaÿ, P. A synthetic heparan sulfate pentasaccharide, exclusively containing l-iduronic acid, displays higher affinity for FGF-2 than its d-glucuronic acid-containing isomers. Bioorg. Med. Chem. 1999, 7, 1567. [Google Scholar]
- Hricovini, M.; Guerrini, M.; Bisio, A.; Torri, G.; Naggi, A.; Casu, B. Active conformations of glycosaminoglycans. NMR determination of the conformation of heparin sequences complexed with antithrombin and fibroblast growth factors in solution. Semin. Thromb. Hemost. 2002, 28, 325–334. [Google Scholar] [CrossRef]
- Guerrini, M.; Hricovíni, M.; Torri, G. Interaction of heparins with fibroblast growth factors: Conformational aspects. Curr. Pharm. Des. 2007, 13, 2045–2056. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Gerwig, G.J.; Vliegenthart, J.F.; Clamp, J.R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem. J. 1975, 151, 491–495. [Google Scholar]
- Montreuil, J.; Bouquelet, S.; Debray, H.; Fournet, B.; Spick, G.; Strecker, G. Glycoproteins. In Carbohydrate Analysis, a Practical Approach; Chaplin, M.F., Kennedy, J.F., Eds.; IRL Press: Oxford, UK, 1986; pp. 143–204. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 4, 447–455. [Google Scholar] [CrossRef]
- Miller, C.C.; Godeau, G.; Lebreton-DeCoster, C.; Desmoulière, A.; Pellat, B.; Dubertret, L. Validation of a morphometric method for evaluating fibroblast numbers in normal and pathologic tissues. Exp. Dermatol. 2003, 12, 403–411. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues. Mar. Drugs 2013, 11, 1351-1369. https://doi.org/10.3390/md11041351
Senni K, Gueniche F, Changotade S, Septier D, Sinquin C, Ratiskol J, Lutomski D, Godeau G, Guezennec J, Colliec-Jouault S. Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues. Marine Drugs. 2013; 11(4):1351-1369. https://doi.org/10.3390/md11041351
Chicago/Turabian StyleSenni, Karim, Farida Gueniche, Sylvie Changotade, Dominique Septier, Corinne Sinquin, Jacqueline Ratiskol, Didier Lutomski, Gaston Godeau, Jean Guezennec, and Sylvia Colliec-Jouault. 2013. "Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues" Marine Drugs 11, no. 4: 1351-1369. https://doi.org/10.3390/md11041351
APA StyleSenni, K., Gueniche, F., Changotade, S., Septier, D., Sinquin, C., Ratiskol, J., Lutomski, D., Godeau, G., Guezennec, J., & Colliec-Jouault, S. (2013). Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues. Marine Drugs, 11(4), 1351-1369. https://doi.org/10.3390/md11041351