2.1. Fucoidan Inhibits Cell Growth and Induces Apoptosis in Leukemic Cells
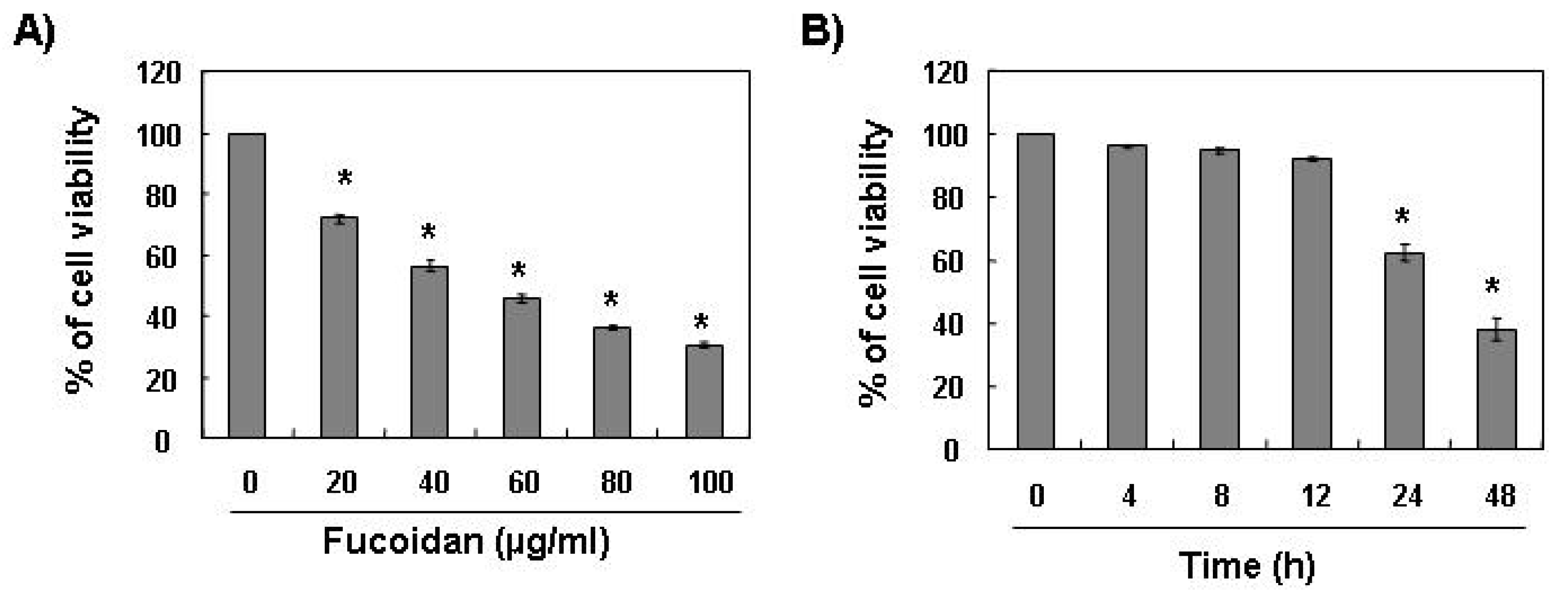
To investigate the effect of fucoidan on cell growth of leukemic cells, U937 cells were exposed to various concentrations of fucoidan for 48 h or 80 μg/mL of fucoidan for the various times points, and cell viability was then measured by the MTT assay. As shown in
Figure 1, treatment with fucoidan decreased the viability of U937 cells in a concentration- and time-dependent manner.
Figure 1.
Effects of fucoidan on cell viability in U937 cells. U937 cells were plated at a concentration of 2.5 × 105 cells in 6-well plates. Following 24 h of stabilization, cells were treated with the indicated concentrations of fucoidan for 48 h (A) or 80 μg/mL of fucoidan for the indicated times (B). The cell viability was measured by the metabolic-dye-based MTT assay. Results are expressed as percentage of the vehicle treated control ± standard deviation (SD) of three separate experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control).
Figure 1.
Effects of fucoidan on cell viability in U937 cells. U937 cells were plated at a concentration of 2.5 × 105 cells in 6-well plates. Following 24 h of stabilization, cells were treated with the indicated concentrations of fucoidan for 48 h (A) or 80 μg/mL of fucoidan for the indicated times (B). The cell viability was measured by the metabolic-dye-based MTT assay. Results are expressed as percentage of the vehicle treated control ± standard deviation (SD) of three separate experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control).
The next experiments were performed to determine if this inhibitory effect of fucoidan on cell viability resulted from apoptotic cell death. To examine apoptosis morphologically, the nuclei of untreated and fucoidan-treated cells were stained with 4,6-diamidino-2-phenyllindile (DAPI) solution and then observed. The control cells displayed intact nuclear structure while cells treated with fucoidan had apoptotic morphological characteristics, such as chromatin condensation and nuclear fragmentation in U937 cells (
Figure 2A). In addition, nucleosomal DNA ladder formation by agarose gel electrophoresis was observed in U937 cells treated with over 40 μg/mL of fucoidan for 48 h (
Figure 2B). We further quantified the degree of apoptotic dead cells by cell cycle analysis. As indicated in
Figure 2C, fucoidan treatment resulted in a significantly increased accumulation of U937 cells at the apoptotic sub-G1 phase and that this response occurred in a concentration-dependent manner.
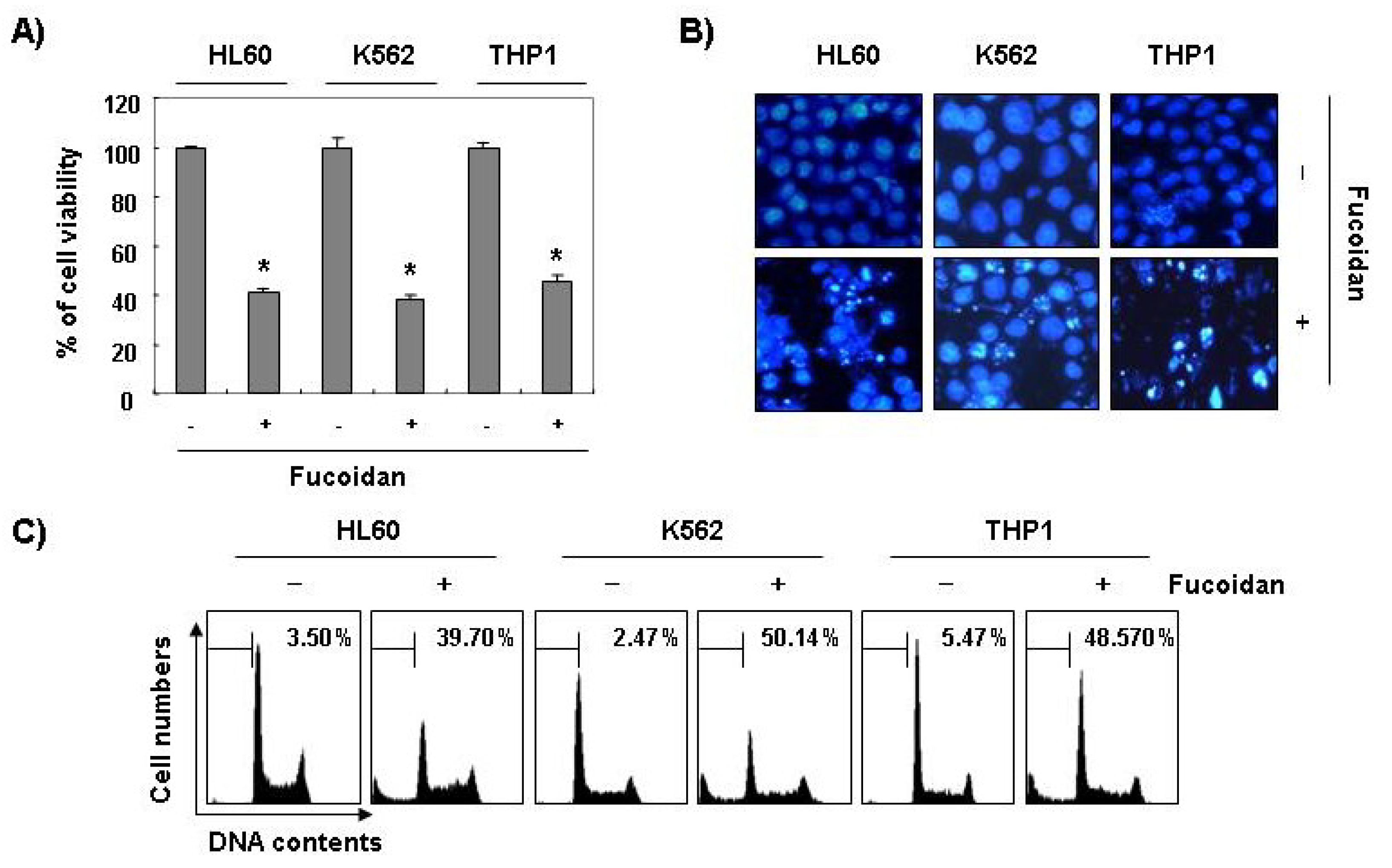
Furthermore, fucoidan significantly inhibited cell viability and induced apoptosis in other leukemic cell lines, such as HL60, K562, and THP1 (
Figure 3). These results demonstrated an association between the growth inhibition observed in response to fucoidan and the induction of apoptosis in leukemic cells.
Figure 2.
Induction of apoptosis by fucoidan treatment in U937 cells. (A) Following 24 h of stabilization, cells were incubated with various concentrations of fucoidan for 48 h. The cells were fixed and stained with DAPI solution. The stained nuclei were then observed under a fluorescent microscope (×400); (B) For the analysis of DNA fragmentation, genomic DNA from cells was extracted, separated by 2.0% agarose gel electrophoresis, and visualized under UV light after staining with EtBr. Marker indicates a size marker of the DNA ladder; (C) To quantify the degree of apoptosis induced by fucoidan, cells were evaluated by flow cytometry for sub-G1 DNA content (hypodiploid DNA), which represents the cells undergoing apoptotic DNA degradation. Data are the mean ± SD of two different experiments.
Figure 2.
Induction of apoptosis by fucoidan treatment in U937 cells. (A) Following 24 h of stabilization, cells were incubated with various concentrations of fucoidan for 48 h. The cells were fixed and stained with DAPI solution. The stained nuclei were then observed under a fluorescent microscope (×400); (B) For the analysis of DNA fragmentation, genomic DNA from cells was extracted, separated by 2.0% agarose gel electrophoresis, and visualized under UV light after staining with EtBr. Marker indicates a size marker of the DNA ladder; (C) To quantify the degree of apoptosis induced by fucoidan, cells were evaluated by flow cytometry for sub-G1 DNA content (hypodiploid DNA), which represents the cells undergoing apoptotic DNA degradation. Data are the mean ± SD of two different experiments.
Figure 3.
Inhibition of cell viability and induction of apoptosis by fucoidan in other leukemic cells. Three leukemic cell lines (HL60, K562, and THP1) were treated with 80 μg/mL fucoidan for 48 h. (A) The cell viability was measured by the metabolic-dye-based MTT assay. Each point represents the mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control); (B) The cells were stained with DAPI solution and stained nuclei were then observed under a fluorescent microscope (×400); (C) The percentage of cells with hypodiploid DNA (sub-G1 phase) were measure by flow cytometry. Each point represents the mean of two independent experiments.
Figure 3.
Inhibition of cell viability and induction of apoptosis by fucoidan in other leukemic cells. Three leukemic cell lines (HL60, K562, and THP1) were treated with 80 μg/mL fucoidan for 48 h. (A) The cell viability was measured by the metabolic-dye-based MTT assay. Each point represents the mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control); (B) The cells were stained with DAPI solution and stained nuclei were then observed under a fluorescent microscope (×400); (C) The percentage of cells with hypodiploid DNA (sub-G1 phase) were measure by flow cytometry. Each point represents the mean of two independent experiments.
2.2. Fucoidan Induces Activation of Caspases and Inhibits the Levels of IAP Family Proteins in U937 Cells
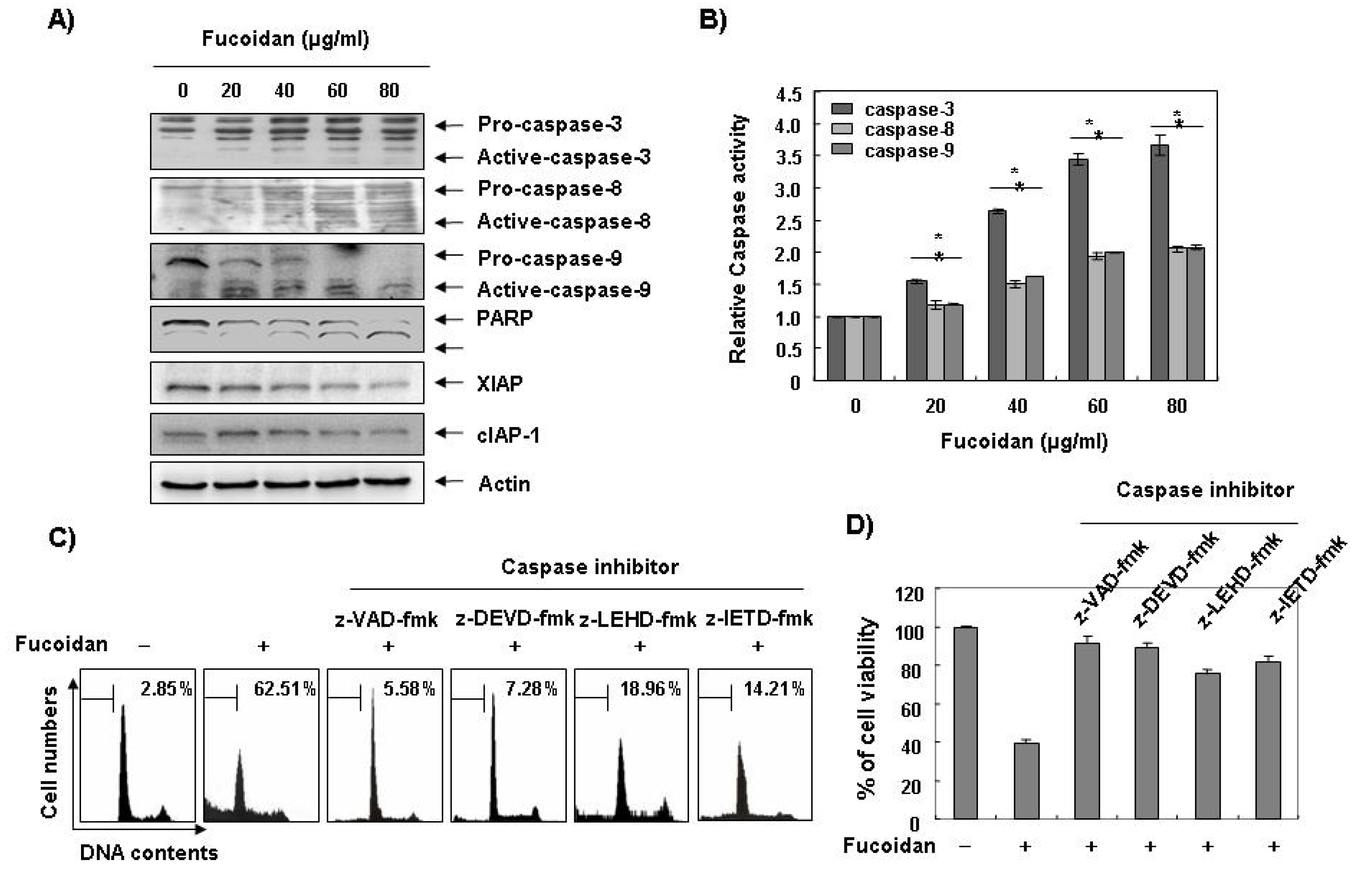
Caspases, known to serve as important mediators of apoptosis in both intrinsic and extrinsic pathway, also contribute to general apoptotic morphology through the cleavage of various cellular substrates, including PARP. Therefore, to gain further insight into the mechanism by which fucoidan induces apoptosis we examined the effects of fucoidan on caspase protein levels and their activities as well as their inhibitor proteins, inhibitor of apoptosis proteins (IAP) family proteins. As
Figure 4A,B reveals, Western blot analyses showed that fucoidan treatment induced an increase in the levels of active-caspase-3, -8, and -9 proteins, and their activities in a concentration-dependent manner. Subsequent Western blot analysis revealed that progressive proteolytic cleavage products of PARP protein and accumulation of the 85 kDa, a downstream target of the activated caspase-3 [
8], occurred in U937 cells treated with fucoidan. In order to demonstrate that the activation of caspases is a key step in the apoptotic pathway induced by fucoidan, U937 cells were pretreated with a pan-caspase inhibitor (z-VAD-fmk) and potential caspase-specific inhibitors (z-DEVD-fmk, z-IETD-fmk, and z-LEHD-fmk for the inactivation of caspase-3, -8, and -9, respectively) for one hour, followed by treatment with fucoidan for 48 h. As shown in
Figure 4C, pretreatment with caspase inhibitors significantly blocked the increase in the sub-G1 population and restored the decreased viability induced by fucoidan (
Figure 4C,D). These results indicate that fucoidan treatment induces apoptosis in U937 cells through a caspase-dependent pathway. Furthermore, fucoidan treatment down-regulated the levels of IAP family proteins, such as XIAP and cIAP-1 (
Figure 4A), which bind to caspases and lead to their inactivation [
25]. These results indicate that fucoidan treatment induces apoptosis through activation of caspases and reduction of IAP family proteins in U937 cells.
Figure 4.
Activation of caspases, degradation of PARP, and inhibition of IAP family proteins by fucoidan in U937 cells. (A) U937 cells were treated with the indicated concentrations of fucoidan for 48 h. The cells were lysed and then cellular proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an enhanced chemiluminescence (ECL) detection system. Actin was used as an internal control; (B) After 48 h incubation with the indicated concentrations of fucoidan, the cells were lysed and aliquots (50 μg protein) were assayed for in vitro caspase-3, -8, and -9 activity using DEVD-pNA, IETD-pNA, and LEHD-pNA as substrates, respectively, at 37 °C for one hour. The released fluorescent products were measured. Data are expressed as mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control); (C and D) The cells were incubated with or without 80 μg/mL fucoidan for 48 h after one hour pretreatment with or without the indicated caspase specific inhibitors (50 μM; z-VAD-fmk, pan-caspase inhibitor; z-DEVD-fmk, caspase-3 inhibitor; z-LEHD-fmk, caspase-9 inhibitor and z-IETD-fmk, caspase-8 inhibitor) and then DNA contents were analyzed by a flow cytometer (C). Each point represents the average of two independent experiments. The degree of growth inhibition was determined by MTT assay (D).
Figure 4.
Activation of caspases, degradation of PARP, and inhibition of IAP family proteins by fucoidan in U937 cells. (A) U937 cells were treated with the indicated concentrations of fucoidan for 48 h. The cells were lysed and then cellular proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an enhanced chemiluminescence (ECL) detection system. Actin was used as an internal control; (B) After 48 h incubation with the indicated concentrations of fucoidan, the cells were lysed and aliquots (50 μg protein) were assayed for in vitro caspase-3, -8, and -9 activity using DEVD-pNA, IETD-pNA, and LEHD-pNA as substrates, respectively, at 37 °C for one hour. The released fluorescent products were measured. Data are expressed as mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*p < 0.05 vs. untreated control); (C and D) The cells were incubated with or without 80 μg/mL fucoidan for 48 h after one hour pretreatment with or without the indicated caspase specific inhibitors (50 μM; z-VAD-fmk, pan-caspase inhibitor; z-DEVD-fmk, caspase-3 inhibitor; z-LEHD-fmk, caspase-9 inhibitor and z-IETD-fmk, caspase-8 inhibitor) and then DNA contents were analyzed by a flow cytometer (C). Each point represents the average of two independent experiments. The degree of growth inhibition was determined by MTT assay (D).
![Marinedrugs 11 02347 g004]()
2.3. Fucoidan Induces Loss of Mitochondrial Membrane Potential (MMP) and Modulation of Bcl-2 Family Members in U937 Cells
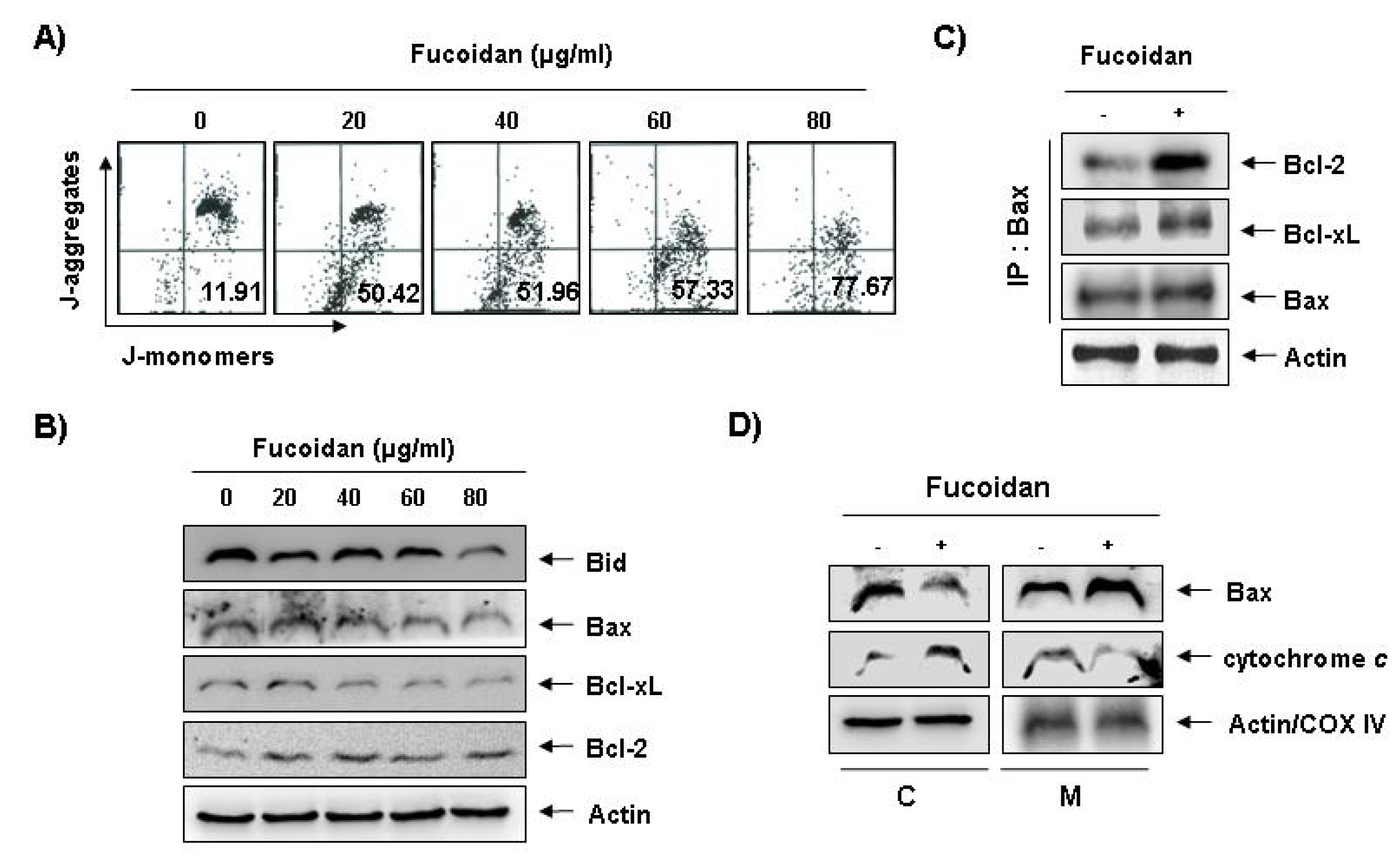
To investigate whether fucoidan-induced apoptosis in U937 cells involves mitochondrial pathway, we next examined the levels of MMP values and Bcl-2 family proteins by a flow cytometer after staining with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1), a fluorescent cationic dye, and Western blot analysis, respectively. As
Figure 5A shows, fucoidan treatment caused a concentration-dependent loss of MMP compared with untreated control. In addition, although we did not detect the truncated form of pro-apoptotic protein Bid, fucoidan decreased the whole form of Bid proteins (
Figure 5B). In addition, while the level of anti-apoptotic Bcl-xL protein decreased concentration-dependency in fucoidan-treated U937 cells, anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins, which function as critical proteins to maintain the stabilization of mitochondria, slightly increased and decreased in response to fucoidan treatment, respectively (
Figure 5B). This result signified an opposite outcome to that found in other studies, because researchers generally believe that Bax increases during the apoptotic process. Further, by and large, we have known Bcl-2 protein to inhibit apoptosis by binding with pro-apoptotic Bax in mitochondria. Therefore, the authors additionally investigated whether or not Bax translocated from cytosol to mitochondria after fucoidan treatment by Co-IP assay and mitochondria fraction. As indicated in
Figure 5C, fucoidan treatment significantly increased the interactions between Bax and Bcl-2, as well as Bax and Bcl-xL. Significantly, we found the binding between Bax and Bcl-2 stronger than that of Bax and Bcl-xL. Furthermore, fucoidan treatment also decreased cytosolic levels of Bax and increased cytosolic levels of cytochrome
c, while mitochondrial levels of Bax significantly increased, and mitochondrial levels of cytochrome
c significantly decreased, respectively (
Figure 5 D). These results suggest that fucoidan inserts Bax from cytosol into mitochondria inducing increased binding between Bax and Bcl-2, and loss of MMP resulting in mitochondrial dysfunction, release of cytochrome
c to cytosol and apoptosis induction.
Figure 5.
Effects of fucoidan on levels of mitochondria membrane potential (MMP) values and Bcl-2 family proteins, and Bax translocation to mitochondria in U937 cells. (A) Ollowing 24 h of stabilization, U937 cells were treated with the indicated concentrations of fucoidan for 48 h. Cells were collected and incubated with JC-1 (10 μM) for 20 min at 37 °C in the dark. The cells were the washed once with phosphate buffered saline (PBS) and analyzed by a DNA flow ctometer. The results are expressed as the mean of two independent experiments; (B) The cell lysates obtained from cells grown under the same conditions as (A) were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. The proteins were visualized using an enhanced chemiluminescence (ECL) detection system; (C) After treatment with or without 80 μg/mL fucoidan for 48 h, total cell lysates were immunoprecipitated with anti-Bax antibody, separated on 10% SDS-polyacrylamide gels, and transferred to nitrocellulose. The levels of Bcl-2 and Bcl-xL proteins were detected with anti-Bcl-2 and anti-Bcl-xL antibodies, respectively, and ECL detection. Immunoprecipitation (IP) actin was used as an internal control; (D) The mitochondrial (M) and cytosolic (C) proteins were extracted and analyzed by Western blotting using the indicated antibodies. Actin and cytochrome oxidase 4 (COX4) were used as internal controls for the cytosolic and mitochondrial fractions, respectively.
Figure 5.
Effects of fucoidan on levels of mitochondria membrane potential (MMP) values and Bcl-2 family proteins, and Bax translocation to mitochondria in U937 cells. (A) Ollowing 24 h of stabilization, U937 cells were treated with the indicated concentrations of fucoidan for 48 h. Cells were collected and incubated with JC-1 (10 μM) for 20 min at 37 °C in the dark. The cells were the washed once with phosphate buffered saline (PBS) and analyzed by a DNA flow ctometer. The results are expressed as the mean of two independent experiments; (B) The cell lysates obtained from cells grown under the same conditions as (A) were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. The proteins were visualized using an enhanced chemiluminescence (ECL) detection system; (C) After treatment with or without 80 μg/mL fucoidan for 48 h, total cell lysates were immunoprecipitated with anti-Bax antibody, separated on 10% SDS-polyacrylamide gels, and transferred to nitrocellulose. The levels of Bcl-2 and Bcl-xL proteins were detected with anti-Bcl-2 and anti-Bcl-xL antibodies, respectively, and ECL detection. Immunoprecipitation (IP) actin was used as an internal control; (D) The mitochondrial (M) and cytosolic (C) proteins were extracted and analyzed by Western blotting using the indicated antibodies. Actin and cytochrome oxidase 4 (COX4) were used as internal controls for the cytosolic and mitochondrial fractions, respectively.
![Marinedrugs 11 02347 g005]()
2.4. Activation of p38 Mitogen-Activated Protein Kinase (MAPK) is Involved in Fucoidan-Induced Apoptosis in U937 Cells
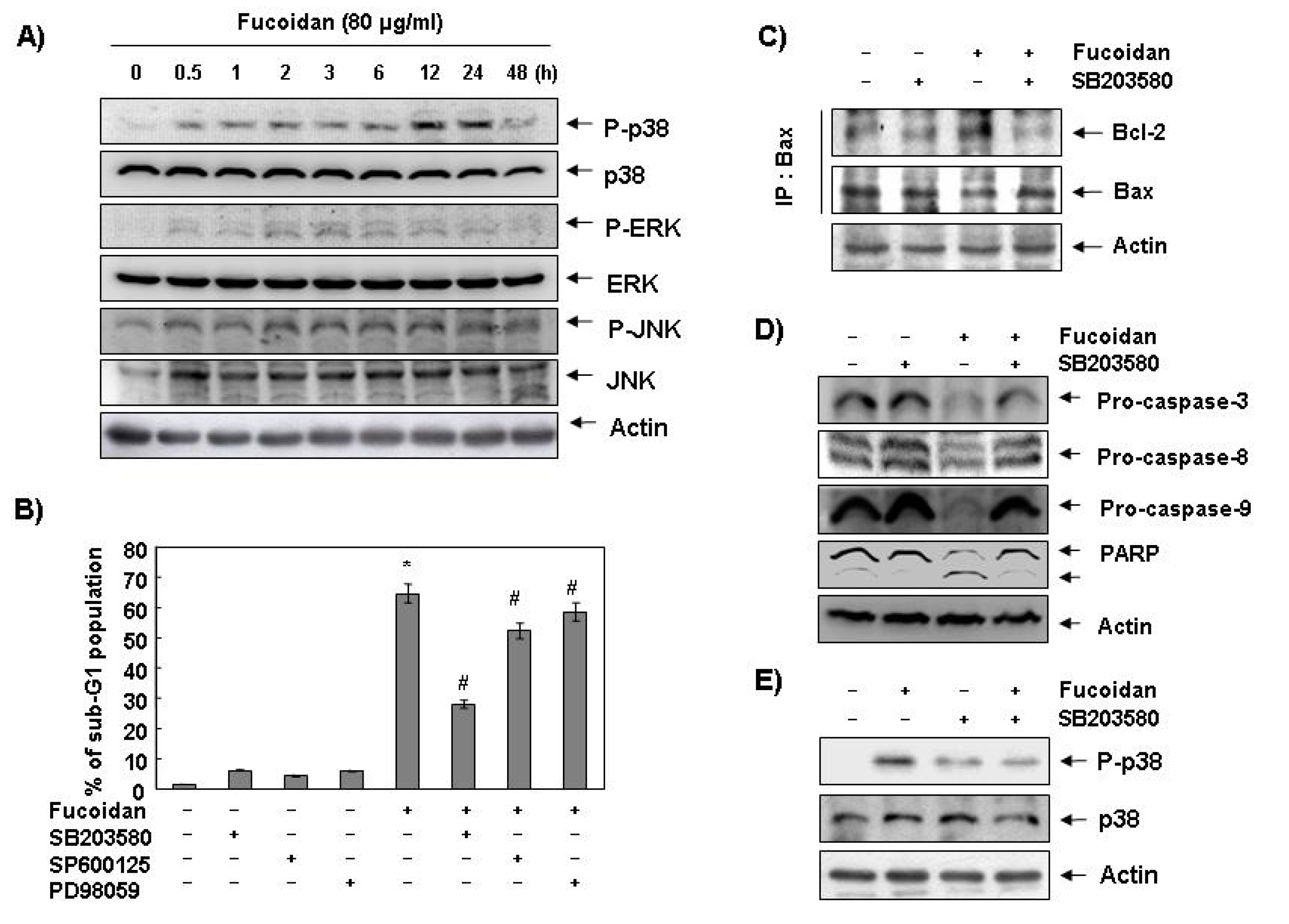
Next, we investigated the effect of fucoidan treatment on the expression and activities of MAPKs to determine if these signaling pathways play a role in mediating the observed apoptotic response. As
Figure 6A demonstrates, the phosphorylated levels of p38 MAPK proteins significantly increased after 12 h and 24 h treatment of fucoidan, compared with ERK and JNK. To confirm an association between the activation of p38 MAPK and the apoptosis induction by fucoidan, we pretreated the cells with MAPK inhibitors and analyzed the sub-G1 DNA content by a flow cytometer. As shown in
Figure 6B, pretreatment with SB203589 (a specific inhibitor of p38 MAPK) significantly reduced the increased number of cells with the sub-G1 DNA content by fucoidan. However, pretreatment with PD98059 (a potent inhibitor of ERK) or SP600125 (a potent inhibitor of JNK) did not have a significant effect on the fucoidan treatment indicating a close involvement of the activation of p38 MAPK with fucoidan-induced apoptosis in U937 cells.
Figure 6.
Effects of p38 MAPK activation on fucoidan-induced apoptosis in U937 cells. (A) Cells were treated with fucoidan (80 μg/mL) for the indicated times. Cells were then lysed, and equal amounts of cell lysates were resolved by SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with the indicated antibodies; (B) Cells were pretreated with the indicated MAPK inhibitors (SB203580 (10 μM), SP600125 (40 μM) and PD98059 (100 μM)) for one hour and then treated with fucoidan (80 μg/mL) for 48 h. The percentage of sub-G1 population was evaluated by a flow cytometer. Each point represents the mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*, p < 0.05 vs. untreated control; #, p < 0.05 present vs. absent MAPK inhibitors); (C) Cells were pretreated with SB203580 (10 μM) for one hour before treatment with 80 μg/mL of fucoidan for 48 h. Bax proteins were immunoprecipitated using ant-Bax antibody, and the immunoprecipitated proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes for Western blot analysis using anti-Bcl-2 and Bax antibodies; (D) Equal amounts of cell lysates extracted from cells were resolved by SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and the proteins were visualized using an ECL detection system; (E) Cells were pretreated with 10 μM SB203580 for one hour and then treated with 80 μg/mL fucoidan for 24 h. They were then lysed, and equal amounts of cell lysates were resolved by SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with the indicated antibodies. Actin was used as an internal control.
Figure 6.
Effects of p38 MAPK activation on fucoidan-induced apoptosis in U937 cells. (A) Cells were treated with fucoidan (80 μg/mL) for the indicated times. Cells were then lysed, and equal amounts of cell lysates were resolved by SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with the indicated antibodies; (B) Cells were pretreated with the indicated MAPK inhibitors (SB203580 (10 μM), SP600125 (40 μM) and PD98059 (100 μM)) for one hour and then treated with fucoidan (80 μg/mL) for 48 h. The percentage of sub-G1 population was evaluated by a flow cytometer. Each point represents the mean ± SD of three independent experiments. The significance was determined by Student’s t-test (*, p < 0.05 vs. untreated control; #, p < 0.05 present vs. absent MAPK inhibitors); (C) Cells were pretreated with SB203580 (10 μM) for one hour before treatment with 80 μg/mL of fucoidan for 48 h. Bax proteins were immunoprecipitated using ant-Bax antibody, and the immunoprecipitated proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes for Western blot analysis using anti-Bcl-2 and Bax antibodies; (D) Equal amounts of cell lysates extracted from cells were resolved by SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and the proteins were visualized using an ECL detection system; (E) Cells were pretreated with 10 μM SB203580 for one hour and then treated with 80 μg/mL fucoidan for 24 h. They were then lysed, and equal amounts of cell lysates were resolved by SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with the indicated antibodies. Actin was used as an internal control.
![Marinedrugs 11 02347 g006]()
2.7. Global Discussion
Although findings from recent studies have demonstrated that fucoidan, a sulfated polysaccharide found in brown algae, can suppress the growthof various cultured human cancer cell lines
in vitro [
19,
20,
21,
22,
23,
24], the signaling pathway by which this occurs remains unclear. The present study aimed to determine the capacity of fucoidan to induce apoptosis and to identify the related biochemical mechanisms in human leukemic cells. The present results clearly demonstrate that fucoidan inhibits leukemic cell growth by induction of apoptotic cell death, which appears to account for its anti-proliferating activity. Measurement of chromatin condensation of the nuclei, DNA fragmentation by agarose gel electrophoresis, and induction of sub-G1 phase by flow cytometry analysis confirmed induction of apoptosis by leukemic cells (
Figure 1,
Figure 2,
Figure 3).
Figure 7.
Effects of Bcl-2 overexpression and Bcl-2 inhibitor on fucoidan-induced apoptosis in U937 cells. (A–C) Control (−, U937/vector) or Bcl-2 transfected (+, U937/Bcl-2) cells were incubated with 80 μg/mL of fucoidan for 48 h. The cells were collected, DNA contents were analyzed by a flow cytometer (A), and the fragmented DNA was extracted and analyzed on a 2.0% agarose gel containing EtBr (C). (B) Equal amounts of cell lysate were extracted, resolved on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with the indicated antibodies. The proteins were visualized using an ECL detection system. Actin was used as an internal loading control. (D) Bcl-2 overexpressing U937 cells were treated with 80 μg/mL of fucoidan in the presence and absence of 15 μM of HA14-1 for 48 h. The percentage of cells with hypodiploid DNA (sub-G1 phase) content represent the fractions undergoing apoptotic DNA degradation. Each point represents the mean of two independent experiments.
Figure 7.
Effects of Bcl-2 overexpression and Bcl-2 inhibitor on fucoidan-induced apoptosis in U937 cells. (A–C) Control (−, U937/vector) or Bcl-2 transfected (+, U937/Bcl-2) cells were incubated with 80 μg/mL of fucoidan for 48 h. The cells were collected, DNA contents were analyzed by a flow cytometer (A), and the fragmented DNA was extracted and analyzed on a 2.0% agarose gel containing EtBr (C). (B) Equal amounts of cell lysate were extracted, resolved on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with the indicated antibodies. The proteins were visualized using an ECL detection system. Actin was used as an internal loading control. (D) Bcl-2 overexpressing U937 cells were treated with 80 μg/mL of fucoidan in the presence and absence of 15 μM of HA14-1 for 48 h. The percentage of cells with hypodiploid DNA (sub-G1 phase) content represent the fractions undergoing apoptotic DNA degradation. Each point represents the mean of two independent experiments.
![Marinedrugs 11 02347 g007]()
Apoptosis plays an important role in the normal development and differentiation of multicellular organisms, characterized by morphological and biological changes such as cytoplasmic shrinkage, chromatin condensation, and DNA degradation [
1]. Apoptosis also serves as a critical protective mechanism against carcinogenesis caused by mutations of genetic materials of normal cells or various carcinogens [
26]. A variety of stimuli can trigger it, including death receptor-mediated signaling (extrinsic pathway) or intracellular stresses (intrinsic pathway) [
1,
27]. Because alterations in mitochondria structure and function, as well as activation of caspase family that has critical roles in regulating apoptosis, initiate apoptosis, we first investigated the catalytic activity of caspases and mitochondrial dysfunction to gain insight into the process of fucoidan-induced apoptosis. The present data revealed that fucoidan reduced the levels of pro-caspase-8 and -9, and increased their catalytic activity, which involves initiator caspases of extrinsic and intrinsic pathways, respectively, in U937 cells (
Figure 4). Fucoidan also down-regulated IAP family proteins, such as XIAP and cIAP-1 (
Figure 4), which reportedly block apoptosis due to their function as direct inhibitors by binding to and inhibiting several caspases. Researchers have considered them to cause resistance to apoptosis in cancer [
25,
28]. Furthermore, fucoidan markedly induced the loss of MMP, an important parameter of mitochondrial function used as an indicator of cell condition, and activated the key executioner, caspase-3, and concomitant degradation of PARP (
Figure 4,
Figure 5). However, blocking caspase activity by pretreating the cells with a pan-caspase inhibitor and caspase specific inhibitors, significantly prevented fucoidan-induced apoptosis and growth inhibition (
Figure 4). Therefore, the data suggest that fucoidan-induced apoptosis in both the U937 cells is caspase-dependent, and the apoptotic effects of fucoidan appear to involve activation of both the intrinsic and extrinsic pathways.
In the mitochondrial pathway, the ratio of expression of the pro-apoptotic proteins such as Bax and the anti-apoptotic proteins such as Bcl-2 and Bcl-xL ultimately determines cell death or survival through regulation of mitochondrial permeability transition. In addition, caspase-8 mediates the intrinsic pathway via cleavage of the pro-apoptotic Bid protein, a BH3-only protein, to a truncated Bid (tBid) through translocation from the cytosol to the mitochondria, triggering mitochondrial dysfunction, followed by activation of caspase-9. This leads to activation of caspase-3 for induction of apoptosis via a release of cytochrome
c to cytosol after translocation of tBid to the mitochondria [
4,
29,
30]. Translocation of pro-apoptotic Bax proteins from cytosol to mitochondria also represents a key event for the activation of apoptosis. The inserted Bax in mitochondria exists as dimers or oligomers, whereas in the cytosol Bax exists as monomers [
31], and the release of cytochrome
c requires mitochondrial membrane insertion and oligomerization of Bax [
32]. Our results indicated that fucoidan treatment induced reduction of whole Bid proteins, which may relate to the activation of tBid (
Figure 5B). Results from Co-IP and mitochondria fraction assays demonstrated that the binding activity between Bcl-2 and Bax proteins, and the levels of Bax proteins in mitochondria and the release of cytochrome
c from mitochondria to cytosol increased (
Figure 5C,D). The data clearly indicate that fucoidan induced Bax translocation to mitochondria from cytosol, leading to the release of cytochrome
c, apoptosome formation, and finally induction of apoptosis in U937 cells.
On the other hand, the MAPKs including, for example, ERK, JNK, and p38 MAPK play critical roles in cell survival and apoptosis in various cancer cells. We know that the activation of the p38 MAPK and JNK pathways leads to induction of apoptosis, whereas we more often associate the ERK with cell survival [
33,
34]. Notably, researchers have reported that p38 MAPK regulates the translocation of Bax from cytosol to the mitochondria in response to a variety of stimuli leading to an apoptotic cell death [
35]. The data mean that activation of p38 MAPK can induce mitochondrial dysfunction and subsequently release apoptogenic proteins such as cytochrome
c from the mitochondria to cytosol, and finally caspase-9 and -3 become activated. In this report, treatment with fucoidan resulted in up-regulation of the p38 MAPK phosphorylation, as opposed to ERK and JNK signaling pathways. Therefore, we investigated the involvement of activation of p38 MAPK signaling pathway in fucoidan-induced apoptosis in U937 cells. As the results indicate, SB203580, specific inhibitor of p38 MAPK, markedly inhibited fucoidan-induced apoptosis of U937 cells by inhibiting the interaction between Bcl-2 and Bax and caspases activation (
Figure 6) suggesting an association of fucoidan-induced apoptosis with Bax translocation to mitochondria via activation of p38 MAPK.
Interestingly, our results indicate pro-apoptotic Bax and anti-apoptotic Bcl-2 levels slightly decreased and increased in response to fucoidan treatment, respectively (
Figure 5B). However, through mitochondrial fractionation assay, we observed that the levels of Bax proteins increased in the mitochondria and decreased in the cytosol, while cytochrome
c represented the reverse tendency (
Figure 5D), which correlated with the increased complex formation between Bax and Bcl-2 (
Figure 5C). We therefore investigated if Bcl-2 overexpression confers protection against fucoidan-induced apoptosis in U937 cells. The results of our study indicate that ectopic Bcl-2 overexpression can block fucoidan-induced caspases activation, cleavage of Bid, as well as PARP and DNA fragmentation to inhibit fucoidan-mediated apoptosis (
Figure 7A–C). Because strategies to overcome Bcl-2-mediated resistance to apoptosis have the potential to greatly increase treatment efficacy, we next examined the ability of a small molecule Bcl-2 inhibitor, HA14-1 [
36], which prevents Bcl-2 interaction with Bax, leading to apoptotic cell death [
37], to determine if it could reverse the anti-apoptotic effect of Bcl-2 and enhance fucoidan-mediated apoptosis. As
Figure 7D shows, Bcl-2 overexpression U937 cells synergistically incubated with HA14-1 and fucoidan had significantly greater levels of apoptotic cells than those treated with fucoidan alone, indicating a close relationship of Bcl-2 to the resistance of fucoidan-mediated apoptosis.
Taken together, these results suggest that activation of p38 MAPK signaling pathway participate in fucoidan-induced apoptosis and that reduction in MMP through modulation of Bcl-2 family proteins is important in fucoidan-induced apoptosis in U937 cells.