Simple and Rapid Quality Control of Sulfated Glycans by a Fluorescence Sensor Assay—Exemplarily Developed for the Sulfated Polysaccharides from Red Algae Delesseria sanguinea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polymer-H Assay for Detection and Quantification of D.s.-SP
2.1.1. Applicability of the Polymer-H Assay

2.1.2. Selection of Calibrator and Reference Substance

2.2. Polymer-H Assay for the Quality Control of D.s.-SP Evaluated by Means of a Large Series of D.s.-SP Batches
2.2.1. Investigation of D.s.-SP Isolated from D.s. Harvested at Different Habitats

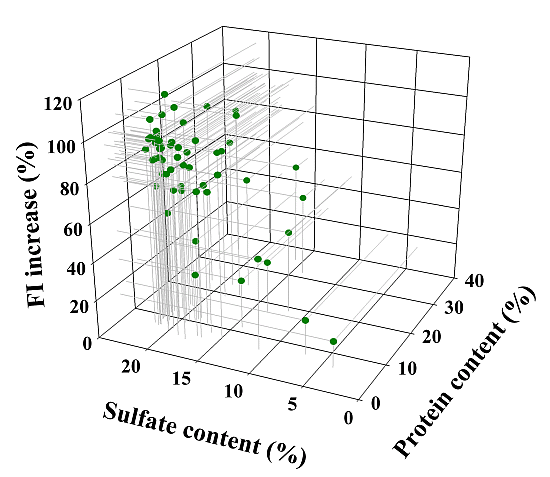
2.2.2. Influence of Sulfate and Protein Content of 57 D.s.-SP Batches on Their FI Increasing Effect

2.2.3. Polymer-H Assay to Check D.s.-SP for Instability, Contamination and Co-Extracted Starch
| Extract | Floridean starch (%) | Polymer-H FI increase (%) |
|---|---|---|
| 1st | 8.2 | 105.9 ± 8.3 |
| 2nd | 9.8 | 98.1 ± 4.8 |
| 3rd | 13.1 | 85.8 ± 2.1 |
| 4th | 15.9 | 84.4 ± 0.4 |
2.2.4. Evaluation of Appropriate FI Increase Limits for the Quality Control of D.s.-SP Batches

| FI increase (%) | ≤70 | >70 | ≤75 | >75 | ≤80 | >80 | ≤85 | >85 | ≤90 | >90 |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 42 | 18 | 39 | 23 | 34 | 31 | 26 | 35 | 22 |
| Mean ± SD | 15.3 ± 5.3 | 20.1 ± 1.8 | 15.7 ± 4.9 | 20.2 ± 1.7 | 16.4 ± 4.6 | 20.4 ± 1.6 | 17.3 ± 4.3 | 20.7 ± 1.3 | 17.6 ± 4.2 | 20.7 ± 1.3 |
| Median | 15.8 | 20.6 | 16.8 | 20.9 | 17.2 | 21.0 | 18.6 | 21.1 | 18.8 | 21.2 |
| 10th percentile | 5.8 | 17.3 | 6.8 | 17.9 | 8.7 | 18.2 | 11.2 | 18.3 | 11.5 | 18.1 |
| 90th percentile | 21.6 | 21.7 | 21.5 | 21.7 | 21.5 | 21.8 | 21.6 | 21.7 | 21.6 | 21.9 |
| FI increase (%) | ≤70 | >70 | ≤75 | >75 | ≤80 | >80 | ≤85 | >85 | ≤90 | >90 |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 13 | 42 | 16 | 39 | 21 | 34 | 29 | 26 | 33 | 22 |
| Mean ± SD | 0.476 ± 0.292 | 0.207 ± 0.053 | 0.430 ± 0.279 | 0.205 ± 0.055 | 0.385 ± 0.255 | 0.199 ± 0.065 | 0.342 ± 0.232 | 0.190 ± 0.039 | 0.321 ± 0.224 | 0.193 ± 0.036 |
| Median | 0.391 | 0.209 | 0.366 | 0.204 | 0.281 | 0.198 | 0.246 | 0.198 | 0.237 | 0.198 |
| 10th percentile | 0.222 | 0.126 | 0.221 | 0.122 | 0.228 | 0.122 | 0.162 | 0.124 | 0.133 | 0.122 |
| 90th percentile | 1.102 | 0.259 | 1.026 | 0.261 | 0.882 | 0.258 | 0.600 | 0.241 | 0.552 | 0.243 |
2.2.5. Verification of the Defined FI Increase Limits by 8 New D.s.-SP Batches

2.3. Polymer-H Assay for Identification of D.s.-SP and Distinction from Other Polysaccharides
2.3.1. Investigation of Other Algae Polysaccharides in the Polymer-H Assay

| Substances | Polymer-H assay | Sulfate content | Elastase inhibition (IC50) | Distinction from D.s.-SP by additional parameters | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean FI increase at 7.5 µg/mL (%) | Linearity 1 (1.0 to 7.5 µg/mL) | slope of the line 2 (rel. to D.s.-SPref) | Distinction from D.s.-SP | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | (% m/m) | (µg/mL) | PolyH/other | |
| D.s.-SP | 80–105 | yes | 1.00 ± 0.11 | - | 20.4 ± 1.6 | 0.20 ± 0.02 | - | ||
| D.s.-SPref | 92 ± 7 | yes | 1.00 | - | 20.5 | 0.19 | - | ||
| Structurally different oligo-and polysaccharides | |||||||||
| Fondaparinux | 110 | no | 2.47 | + | + | + | 41.5 | >10 | + |
| Heparin | 107 | no | 2.06 | + | + | + | 27.7 | 0.31 | + |
| Phycarin | 0 | no | 0.0 | + | + | + | 0.0 | >25 | + |
| Agarose | 0 | no | 0.0 | + | + | + | ≤0.2 | >6.25 | + |
| Fucoidan | 63 | no | 0.68 | + | + | + | 20.8 | 0.69 | + |
| Alginate sodium | 91 | no | 1.47 | − | + | + | 0.0 | >2.5 | + |
| λ-carrageenan | 106 | (yes) | 1.08 | (+) | − | − 3 | 44.0 | 0.22 | + 3/SC |
| κ-carrageenan | 85 | (yes) | 0.85 | − | − | (+) 3 | 20.7 | >6.25 | + 3/IC50 |
| Red algae polysaccharides structurally related to D.s.-SP | |||||||||
| D.s.-SP 7.1 | 93 | yes | 0.92 | − | − | − | 20.2 | 0.19 | - |
| P.r.-SP | 113 | yes | 1.18 | + | − | (+) 3 | 21.0 | 0.19 | + |
| P.p.-SP | 99 | yes | 1.01 | − | − | − | 16.7 | 0.45 | −/SC, IC50 |
| C.t.-SP | 72 | yes | 0.73 | + | − | + | 16.0 | 0.18 | + |
2.3.2. Investigation of Sulfated Xylogalactans from Various Red Algae in the Polymer-H Assay

2.3.3. Test Concept for Identification of D.s.-SP and Distinction from Other Sulfated Glycans
3. Experimental Section
3.1. Polymer-H
3.2. Heparin, Fondaparinux and PS3
3.3. Sulfated Polysaccharides Fraction of Delesseria sanguinea (D.s.-SP)
3.4. Other Algae Polysaccharides
3.5. Polymer-H Fluorescence Assay
3.6. Analytical Methods
3.7. Elastase Activity Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar]
- Kirn-Safran, C.; Farach-Carson, M.C.; Carson, D.D. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell. Mol. Life Sci. 2009, 66, 3421–3434. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar]
- Alban, S. Natural and synthetic glycosaminoglycans. Molecular characteristics as the basis of distinct drug profiles. Hämostaseologie 2008, 28, 51–61. [Google Scholar]
- Gandhi, N.S.; Mancera, R.L. Heparin/heparan sulphate-based drugs. Drug Discov. Today 2010, 15, 1058–1069. [Google Scholar] [CrossRef]
- Pomin, V.H. Structure-function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Elsevier: Waltham, MA, USA, 2012; Vol. 65, Chapter 12; pp. 195–209. [Google Scholar]
- Pomin, V.H. Fucanomics and galactanomics: Marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef]
- Vilela-silva, A.-C.E.; Hirohashi, N.; Mouro, P.A. The structure of sulfated polysaccharides ensures a carbohydrate-based mechanism for species recognition during sea urchin fertilization. Int. J. Dev. Biol. 2008, 52, 551–559. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Aquino, R.S.; Grativol, C.; Mourão, P.A.S.; Meyer, P. Rising from the Sea: Correlations between Sulfated Polysaccharides and Salinity in Plants. PLoS One 2011, 6, e18862. [Google Scholar]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 489–532. [Google Scholar]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Vo, T.-S.; Kim, S.-K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Grünewald, N.; Groth, I.; Alban, S. Evaluation of seasonal variations of the structure and anti-inflammatory activity of sulfated polysaccharides extracted from the red alga Delesseria sanguinea (Hudson) Lamouroux (Ceramiales, Delesseriaceae). Biomacromolecules 2009, 10, 1155–1162. [Google Scholar] [CrossRef]
- Grünewald, N.; Alban, S. Optimized and standardized isolation and structural characterization of anti-inflammatory sulfated polysaccharides from the red alga Delesseria sanguinea (Hudson) Lamouroux (Ceramiales, Delesseriaceae). Biomacromolecules 2009, 10, 2998–3008. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Groth, I.; Grunewald, N.; Alban, S. Pharmacological profiles of animal- and nonanimal-derived sulfated polysaccharides—Comparison of unfractionated heparin, the semisynthetic glucan sulfate PS3, and the sulfated polysaccharide fraction isolated from Delesseria sanguinea. Glycobiology 2009, 19, 408–417. [Google Scholar] [CrossRef]
- Alban, S.; Lühn, S.; Schiemann, S.; Beyer, T.; Norwig, J.; Schilling, C.; Radler, O.; Wolf, B.; Matz, M.; Baumann, K.; et al. Comparison of established and novel purity tests for the quality control of heparin by means of a set of 177 heparin samples. Anal. Bioanal. Chem. 2011, 399, 605–620. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar] [CrossRef]
- Huang, S.; Jia, M.; Xie, Y.; Wang, J.; Xu, W.; Fang, H. The progress of selective fluorescent chemosensors by boronic acid. Curr. Med. Chem. 2012, 19, 2621–2637. [Google Scholar] [CrossRef]
- Cao, H.; Heagy, M.D. Fluorescent chemosensors for carbohydrates: A decade’s worth of bright spies for saccharides in review. J. Fluoresc. 2004, 14, 569–584. [Google Scholar] [CrossRef]
- Lühn, S.; Schrader, T.; Sun, W.; Alban, S. Development and evaluation of a fluorescence microplate assay for quantification of heparins and other sulfated carbohydrates. J. Pharm. Biomed. Anal. 2010, 52, 1–8. [Google Scholar] [CrossRef]
- Sun, W.; Bandmann, H.; Schrader, T. A fluorescent polymeric heparin sensor. Chemistry 2007, 13, 7701–7707. [Google Scholar] [CrossRef]
- Wright, A.T.; Zhong, Z.; Anslyn, E.V. A functional assay for heparin in serum using a designed synthetic receptor. Angew. Chem. Int. Ed. Engl. 2005, 44, 5679–5682. [Google Scholar] [CrossRef]
- Alban, S. From heparins to factor Xa inhibitors and beyond. Eur. J. Clin. Investig. 2005, 35, S12–S20. [Google Scholar] [CrossRef]
- Schiemann, S.; Lühn, S.; Alban, S. Development of both colorimetric and fluorescence heparinase activity assays using fondaparinux as substrate. Anal. Biochem. 2012, 427, 82–90. [Google Scholar] [CrossRef]
- Heparin Sodium. In European Pharmacopoeia, 8th ed.; EDQM Council of Europe: Strasbourg, France, 2010.
- Alginic Acid. In European Pharmacopoeia, 7th ed.; EDQM Council of Europe: Strasbourg, France, 2009.
- Yvin, J.; Alban, S.; Franz, G. Anti-Inflammatory and Healing Medicine Based on Laminarin Sulphate. PCT International Application Patent No WO 2002036132, 10 May 2002. [Google Scholar]
- Alban, S.; Ludwig, R.J.; Bendas, G.; Schon, M.P.; Oostingh, G.J.; Radeke, H.H.; Fritzsche, J.; Pfeilschifter, J.; Kaufmann, R.; Boehncke, W.H. PS3, a semisynthetic beta-1,3-glucan sulfate, diminishes contact hypersensitivity responses through inhibition of L- and P-selectin functions. J. Investig. Dermatol. 2009, 129, 1192–1202. [Google Scholar] [CrossRef]
- Groth, I.; Alban, S. Elastase inhibition assay with peptide substrates—An example for the limited comparability of in vitro results. Planta Med. 2008, 74, 852–858. [Google Scholar] [CrossRef]
- Piraud, M.; Maire, I.; Mathieu, M. Pitfalls of screening for mucopolysaccharidoses by the dimethylmethylene blue test. Clin. Chem. 1993, 39, 163–164. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lühn, S.; Grimm, J.C.; Alban, S. Simple and Rapid Quality Control of Sulfated Glycans by a Fluorescence Sensor Assay—Exemplarily Developed for the Sulfated Polysaccharides from Red Algae Delesseria sanguinea. Mar. Drugs 2014, 12, 2205-2227. https://doi.org/10.3390/md12042205
Lühn S, Grimm JC, Alban S. Simple and Rapid Quality Control of Sulfated Glycans by a Fluorescence Sensor Assay—Exemplarily Developed for the Sulfated Polysaccharides from Red Algae Delesseria sanguinea. Marine Drugs. 2014; 12(4):2205-2227. https://doi.org/10.3390/md12042205
Chicago/Turabian StyleLühn, Susanne, Juliane C. Grimm, and Susanne Alban. 2014. "Simple and Rapid Quality Control of Sulfated Glycans by a Fluorescence Sensor Assay—Exemplarily Developed for the Sulfated Polysaccharides from Red Algae Delesseria sanguinea" Marine Drugs 12, no. 4: 2205-2227. https://doi.org/10.3390/md12042205
APA StyleLühn, S., Grimm, J. C., & Alban, S. (2014). Simple and Rapid Quality Control of Sulfated Glycans by a Fluorescence Sensor Assay—Exemplarily Developed for the Sulfated Polysaccharides from Red Algae Delesseria sanguinea. Marine Drugs, 12(4), 2205-2227. https://doi.org/10.3390/md12042205