Evolving Marine Biomimetics for Regenerative Dentistry
Abstract
:1. Introduction
1.1. Striving for de Novo Regeneration of Whole Living Tooth Tissues
1.2. Frameworks for Tissue Engineering
| Marine Biostructures | Other Biomaterials and Their Structures |
|---|---|
| Pre-designed and pre-fabricated with numerous possible roles in cell and tissue regeneration. Intrinsic functions can be translated to human biology. | Fabricated by designer with many honed functions specific to biomimetic induction, control and regulation of developmental and regeneration processes. |
| Inherent hierarchical structural design. | Limited levels of hierarchy can be assembled, so far. |
| High diversity of structure and architecture. Some species highly abundant and sustainably sourced. | Structural and architectural specificity is diverse but difficult to generate artificially requiring complicated processing. |
| Intrinsic design features retained for fracture prevention and biorecognition (physical, biochemical, sometimes cellular). | Difficulty in generating composites with comprehensive fracture toughening. |
| Short processing times with low cost. Although obtaining marine biostructures from natural habitats can be expensive. | Long manufacturing process with many steps at high cost. |
| Some difficulties in sterility and removing all contaminants. | High levels of sterility obtained. |
| Safe storage indefinitely usually without special conditions. | Variable storage times with possible degradation. |
| Possibility of low-level immunogenicity. | Synthetics have no immunogenicity. Some natural biopolymers risk immunogenicity. |
| Low cytotoxicity in properly cleaned biostructures. | Low cytotoxicity. |
| Less utility to integrate bioactive elements. | Large range of bioactive elements (adhesive ligands, enzyme-sensitive cross-links) can be integrated biomimetically during synthesis. |
| Less accessible in adding new biomimetic properties. | Highly accessible design and construction with many biomimetic properties: self actuation, self-adjustment, etc. |
2. Biomedical Applications of Marine Products
2.1. Source of Marine Biomaterials and Products


2.2. Marine Products in Dental Tissue Engineering

2.2.1. Tooth Remineralization
2.2.2. Biofilm Prevention and Disruption
2.2.3. Scaffolds and Biostructures
2.2.3.1. Marine Sponge Skeletons

2.2.3.2. Diatom Skeletons

2.2.3.3. Foraminifera Microskeletons

2.2.4. Bone Substitutes
2.2.4.1. Nacre Seashell


2.2.4.2. Biotransformed Coral Skeletons
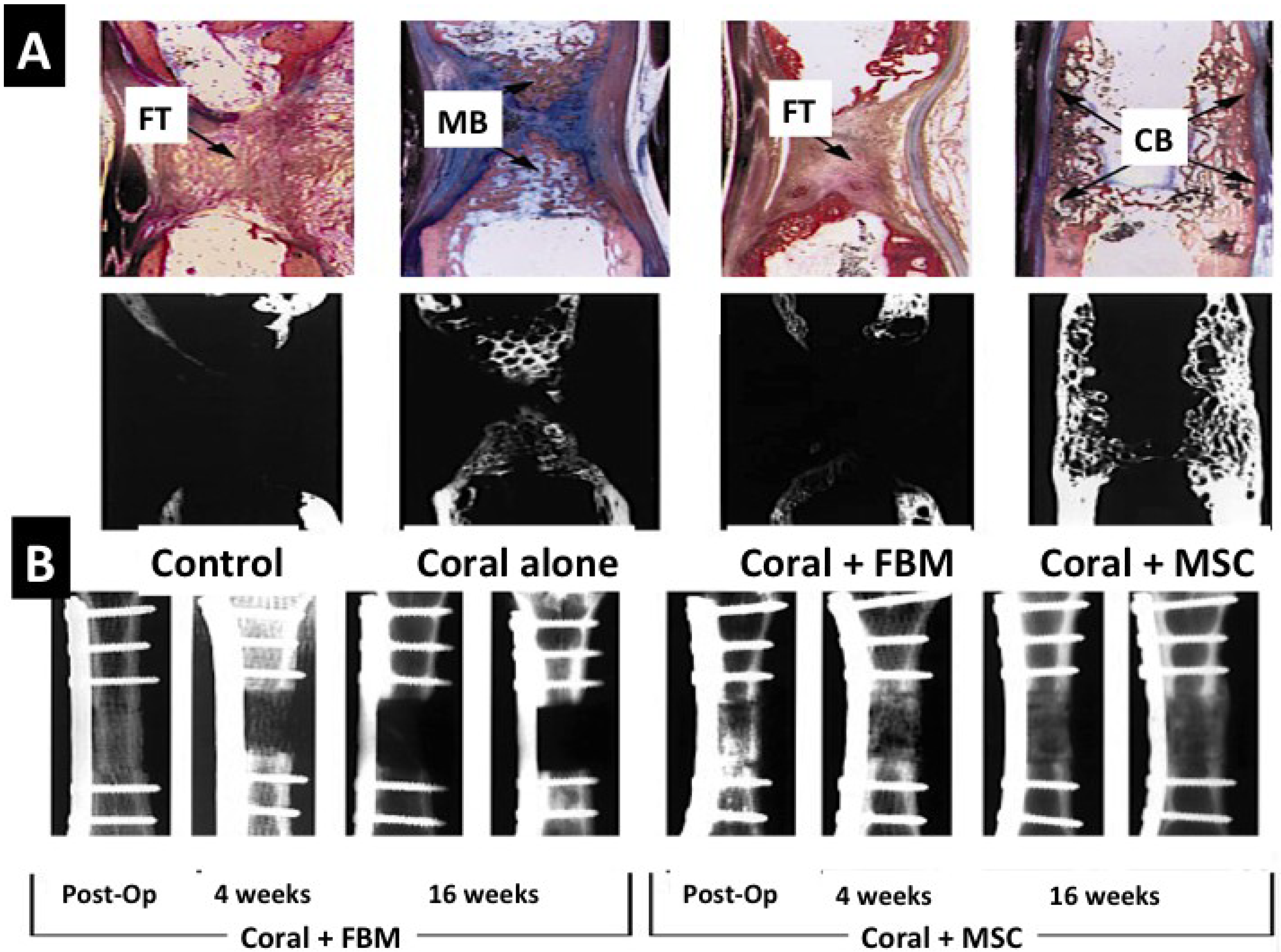

3. Limitations and Challenges
4. Conclusions

Acknowledgments
Conflicts of Interest
References
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Fadeeva, E.; Deiwick, A.; Chichkov, B.; Schlie-Wolter, S. Impact of laser-structured biomaterial interfaces on guided cell responses. Interface Focus 2014, 4, 20130048. [Google Scholar] [CrossRef]
- Vadgama, P. Surfaces and Interfaces for Biomaterials; CRC Press, Woodhead Pubulisher Ltd.: Boca Raton, Cambridge, England, 2005; p. 802. [Google Scholar]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. Biomaterials: A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef]
- Lee, H.; Lytton-Jean, A.K.; Chen, Y.; Love, K.T.; Park, A.I.; Karagiannis, E.D.; Sehgal, A.; Querbes, W.; Zurenko, C.S.; Jayaraman, M.; et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012, 7, 389–393. [Google Scholar] [CrossRef]
- Putnam, D. Design and development of effective siRNA delivery vehicles. Proc. Natl. Acad. Sci. USA 2014, 111, 3903–3904. [Google Scholar] [CrossRef]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef]
- Ohara, T.; Itaya, T.; Usami, K.; Ando, Y.; Sakurai, H.; Honda, M.J.; Ueda, M.; Kagami, H. Evaluation of scaffold materials for tooth tissue engineering. J. Biomed. Mater. Res. A 2010, 94, 800–805. [Google Scholar]
- Soffer, E.; Ouhayoun, J.P.; Anagnostou, F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 521–528. [Google Scholar] [CrossRef]
- Tozum, T.F.; Demiralp, B. Platelet-rich plasma: A promising innovation in dentistry. J. Can. Dent. Assoc. 2003, 69, 664. [Google Scholar]
- Carlson, N.E.; Roach, R.B., Jr. Platelet-rich plasma: Clinical applications in dentistry. J. Am. Dent. Assoc. 2002, 133, 1383–1386. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 117–135. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.O.; Kim, S.H.; Choi, H.J.; Son, H.K.; Jung, H.S.; Kim, C.S.; Lee, J.H. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng. Part A 2012, 18, 2040–2051. [Google Scholar] [CrossRef]
- Bluteau, G.; Luder, H.U.; de Bari, C.; Mitsiadis, T.A. Stem cells for tooth engineering. Eur. Cell Mater. 2008, 16, 1–9. [Google Scholar]
- Ohazama, A.; Tucker, A.; Sharpe, P.T. Organized tooth-specific cellular differentiation stimulated by BMP4. J. Dent. Res. 2005, 84, 603–606. [Google Scholar] [CrossRef]
- Mina, M.; Kollar, E.J. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 1987, 32, 123–127. [Google Scholar] [CrossRef]
- Tucker, A.S.; Matthews, K.L.; Sharpe, P.T. Transformation of tooth type induced by inhibition of BMP signaling. Science 1998, 282, 1136–1138. [Google Scholar] [CrossRef]
- Lumsden, A.G. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 1988, 103, 155–169. [Google Scholar]
- Zhang, Y.D.; Chen, Z.; Song, Y.Q.; Liu, C.; Chen, Y.P. Making a tooth: Growth factors, transcription factors, and stem cells. Cell Res. 2005, 15, 301–316. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Van Nieuwenhuysen, J.P.; D’Hoore, W.; Carvalho, J.; Qvist, V. Long-term evaluation of extensive restorations in permanent teeth. J. Dent. 2003, 31, 395–405. [Google Scholar] [CrossRef]
- Langer, R. Tissue engineering: A new field and its challenges. Pharm. Res. 1997, 14, 840–841. [Google Scholar] [CrossRef]
- Langer, R. Tissue engineering. Mol. Ther. 2000, 1, 12–15. [Google Scholar] [CrossRef]
- Cimetta, E.; Sirabella, D.; Yeager, K.; Davidson, K.; Simon, J.; Moon, R.T.; Vunjak-Novakovic, G. Microfluidic bioreactor for dynamic regulation of early mesodermal commitment in human pluripotent stem cells. Lab Chip 2013, 13, 355–364. [Google Scholar] [CrossRef]
- Harink, B.; Le Gac, S.; Truckenmuller, R.; van Blitterswijk, C.; Habibovic, P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab Chip 2013, 13, 3512–3528. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef]
- Zopf, D.A.; Hollister, S.J.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized formation of polarized cortical tissues from escs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Griffith, C.K.; Miller, C.; Sainson, R.C.; Calvert, J.W.; Jeon, N.L.; Hughes, C.C.; George, S.C. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005, 11, 257–266. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef]
- Green, D.W.; Watson, G.S.; Watson, J.; Abraham, S.J. New biomimetic directions in regenerative ophthalmology. Adv. Healthc. Mater. 2012, 1, 140–148. [Google Scholar] [CrossRef]
- Green, D.W. Tissue bionics: Examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 034010. [Google Scholar] [CrossRef]
- Chou, J.; Valenzuela, S.M.; Santos, J.; Bishop, D.; Milthorpe, B.; Green, D.W.; Otsuka, M.; Ben-Nissan, B. Strontium- and magnesium-enriched biomimetic beta-TCP macrospheres with potential for bone tissue morphogenesis. J. Tissue Eng. Regen. Med. 2012. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Svetličić, V.; Žutić, V.; Radić, T.M.; Pletikapić, G.; Zimmermann, A.H.; Urbani, R. Polymer networks produced by marine diatoms in the northern adriatic sea. Mar. Drugs 2011, 9, 666–679. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Marine Biomaterials: Characterization, Isolation, and Applications; Kim, S.-K., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2013. [Google Scholar]
- Marris, E. Marine natural products: Drugs from the deep. Nature 2006, 443, 904–905. [Google Scholar] [CrossRef]
- Ebada, S.S.; Edrada, R.A.; Lin, W.H.; Proksch, P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1832. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants. Bioorg. Med. Chem. 2011, 19, 6658–6674. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Chiroff, R.T.; White, R.A.; White, E.W.; Weber, J.N.; Roy, D. The restoration of the articular surfaces overlying Replamineform porous biomaterials. J. Biomed. Mater. Res. 1977, 11, 165–178. [Google Scholar] [CrossRef]
- Chiroff, R.T.; White, E.W.; Weber, K.N.; Roy, D.M. Tissue ingrowth of Replamineform implants. J. Biomed. Mater. Res. 1975, 9, 29–45. [Google Scholar] [CrossRef]
- White, R.A.; Weber, J.N.; White, E.W. Replamineform: A new process for preparing porous ceramic, metal, and polymer prosthetic materials. Science 1972, 176, 922–924. [Google Scholar]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of sea urchin spines to Mg-substituted tricalcium phosphate for bone implants. Acta Biomater. 2007, 3, 785–793. [Google Scholar] [CrossRef]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar]
- Varoni, E.M.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Plant polyphenols and oral health: Old phytochemicals for new fields. Curr. Med. Chem. 2012, 19, 1706–1720. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Monaghan, P. Titanium and titanium alloys as dental materials. Int. Dent. J. 1993, 43, 245–253. [Google Scholar]
- Parr, G.R.; Gardner, L.K.; Toth, R.W. Titanium: The mystery metal of implant dentistry. Dental materials aspects. J. Prosthet. Dent. 1985, 54, 410–414. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A.; Al-Sabbagh, M. Bioactive glass three decades on. J. Long Term Eff. Med. Implants 2005, 15, 585–597. [Google Scholar] [CrossRef]
- Lacefield, W.R. Current status of ceramic coatings for dental implants. Implant Dent. 1998, 7, 315–322. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef]
- Sjostrom, T.; Brydone, A.S.; Meek, R.M.; Dalby, M.J.; Su, B.; McNamara, L.E. Titanium nanofeaturing for enhanced bioactivity of implanted orthopedic and dental devices. Nanomedicine (Lond.) 2013, 8, 89–104. [Google Scholar] [CrossRef]
- Tomisa, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.; Saiz, E. Nanotechnology approaches to improve dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25–44; discussion 45–49. [Google Scholar]
- Ure, D.; Harris, J. Nanotechnology in dentistry: Reduction to practice. Dent. Update 2003, 30, 10–15. [Google Scholar]
- Melo, M.A.; Guedes, S.F.; Xu, H.H.; Rodrigues, L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef]
- Clarke, S.A.; Walsh, P.; Maggs, C.A.; Buchanan, F. Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol. Adv. 2011, 29, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A therapeutic potential for marine skeletal proteins in bone regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, A. Farming sponges to supply bioactive metabolites and bath sponges: A review. Mar. Biotechnol. 2009, 11, 669–679. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Jaspars, M.; Hranueli, D.; Battershill, C.N.; Peric-Concha, N.; Zucko, J.; Wright, S.H.; Long, P.F. New methods for medicinal chemistry—Universal gene cloning and expression systems for production of marine bioactive metabolites. Curr. Med. Chem. 2006, 13, 697–710. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Battershill, C.N.; Liptrot, C.H.; Cobb, R.E.; Bourne, D.G.; Jaspars, M.; Long, P.F.; Newman, D.J. Biomedicinals from the phytosymbionts of marine invertebrates: A molecular approach. Methods 2007, 42, 358–376. [Google Scholar] [CrossRef]
- Abramovitch-Gottlib, L.; Geresh, S.; Vago, R. Biofabricated marine hydrozoan: A bioactive crystalline material promoting ossification of mesenchymal stem cells. Tissue Eng. 2006, 12, 729–739. [Google Scholar] [CrossRef]
- Walsh, P.J.; Buchanan, F.J.; Dring, M.; Maggs, C.; Bell, S.; Walker, G.M. Low-pressure synthesis and characterisation of hydroxyapatite derived from mineralise red algae. Chem. Eng. J. 2008, 137, 173–179. [Google Scholar] [CrossRef]
- Walsh, P.J.; Walker, G.M.; Maggs, C.A.; Buchanan, F.J. A study of the relationship between process conditions and mechanical strength of mineralized red algae in the preparation of a marine-derived bone void filler. Proc. Inst. Mech. Eng. H 2011, 225, 563–574. [Google Scholar]
- Dawkins, D. Climbing Mount Improbable; Norton: New York, NY, USA, 1996. [Google Scholar]
- Townley, H.E.; Woon, K.L.; Payne, F.P.; White-Cooper, H.; Parker, A.R. Modification of the physical and optical properties of the frustule of the diatom Coscinodiscus wailesii by nickel sulfate. Nanotechnology 2007, 18, 295101. [Google Scholar] [CrossRef]
- Kroger, N. Prescribing diatom morphology: Toward genetic engineering of biological nanomaterials. Curr. Opin. Chem. Biol. 2007, 11, 662–669. [Google Scholar] [CrossRef]
- Chou, J.; Green, D.W.; Singh, K.; Hao, J.; Ben-Nissan, B.; Milthorpe, B. Adipose stem cell coating of biomimetic beta-tcp macrospheres by use of laboratory centrifuge. Biores. Open Access 2013, 2, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Mann, S.; Ozin, G.A. Synthesis of inorganic materials with complex form. Nature 1996, 382, 313–318. [Google Scholar] [CrossRef]
- Mann, S.; Archibald, D.D.; Didymus, J.M.; Douglas, T.; Heywood, B.R.; Meldrum, F.C.; Reeves, N.J. Crystallization at Inorganic-organic Interfaces: Biominerals and biomimetic synthesis. Science 1993, 261, 1286–1292. [Google Scholar]
- Young, J.R.; Davis, S.A.; Bown, P.R.; Mann, S. Coccolith ultrastructure and biomineralisation. J. Struct. Biol. 1999, 126, 195–215. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Cary, NC, USA, 2001; Volume 5. [Google Scholar]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef]
- Matsumura, G.; Isayama, N.; Matsuda, S.; Taki, K.; Sakamoto, Y.; Ikada, Y.; Yamazaki, K. Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. Biomaterials 2013, 34, 6422–6428. [Google Scholar] [CrossRef]
- Matsumura, G.; Nitta, N.; Matsuda, S.; Sakamoto, Y.; Isayama, N.; Yamazaki, K.; Ikada, Y. Long-term results of cell-free biodegradable scaffolds for tissue-engineering vasculature: In a canine inferior vena cava model. PLoS One 2012, 7, e35760. [Google Scholar]
- Langer, R. Perspectives and challenges in tissue engineering and regenerative medicine. Adv. Mater. 2009, 21, 3235–3236. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sorlie, M.; Varum, K.M.; Eijsink, V.G. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Lieder, R.; Thormodsson, F.; Ng, C.H.; Einarsson, J.M.; Gislason, J.; Petersen, P.H.; Sigurjonsson, O.E. Chitosan and Chitin Hexamers affect expansion and differentiation of mesenchymal stem cells differently. Int. J. Biol. Macromol. 2012, 51, 675–680. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, T.H.; Reis, R.L.; Mano, J.F. Hierarchical fibrillar scaffolds obtained by non-conventional layer-by-layer electrostatic self-assembly. Adv. Healthc. Mater. 2013, 2, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Chen, W.; Mao, F.; Wang, Y. Effect of chitooligosaccharides on mice hematopoietic stem/progenitor cells. Int. J. Biol. Macromol. 2013, 54, 71–75. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Rowley, J.A.; Mooney, D.J. Engineering growing tissues. Proc. Natl. Acad Sci. USA 2002, 99, 12025–12030. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; von Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine collagen scaffolds for nasal cartilage repair: Prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. Natural enamel wear—A physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med. Hypotheses 2010, 74, 670–672. [Google Scholar] [CrossRef]
- Nakashima, S.; Yoshie, M.; Sano, H.; Bahar, A. Effect of a test dentifrice containing nano-sized calcium carbonate on remineralization of enamel lesions in vitro. J. Oral Sci. 2009, 51, 69–77. [Google Scholar] [CrossRef]
- Saunders, S.A. Current practicality of nanotechnology in dentistry. Part 1: Focus on nanocomposite restoratives and biomimetics. Clin. Cosmet. Investig. Dent. 2009, 1, 47–61. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef]
- Li, L.; Mao, C.; Wang, J.; Xu, X.; Pan, H.; Deng, Y.; Gu, X.; Tang, R. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef]
- Uchida, T.; Murakami, C.; Wakida, K.; Dohi, N.; Iwai, Y.; Simmer, J.P.; Fukae, M.; Satoda, T.; Takahashi, O. Sheath proteins: Synthesis, secretion, degradation and fate in forming enamel. Eur. J. Oral Sci. 1998, 106, 308–314. [Google Scholar]
- Jodaikin, A.; Traub, W.; Weiner, S. Protein conformation in rat tooth enamel. Arch. Oral Biol. 1986, 31, 685–689. [Google Scholar] [CrossRef]
- Jodaikin, A.; Weiner, S.; Traub, W. Enamel rod relations in the developing rat incisor. J. Ultrastruct. Res. 1984, 89, 324–332. [Google Scholar] [CrossRef]
- Mann, S. The biomimetics of enamel: A paradigm for organized biomaterials synthesis. Ciba Found. Symp. 1997, 205, 261–269; discussion 269–274. [Google Scholar]
- Li, X.; Pan, D.; Lin, S.; Zhuang, Z.; Lin, Z. Facile in vitro hydroxyapatite remineralization of human enamel with remarkable hardness. CrystEngComm 2013, 15, 4351–4356. [Google Scholar] [CrossRef]
- Mouries, L.P.; Almeida, M.J.; Milet, C.; Berland, S.; Lopez, E. Bioactivity of nacre water-soluble organic matrix from the bivalve mollusk Pinctada maxima in three mammalian cell types: Fibroblasts, bone marrow stromal cells and osteoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 217–229. [Google Scholar] [CrossRef]
- Almeida, M.J.; Pereira, L.; Milet, C.; Haigle, J.; Barbosa, M.; Lopez, E. Comparative effects of nacre water-soluble matrix and dexamethasone on the alkaline phosphatase activity of MRC-5 fibroblasts. J. Biomed. Mater. Res. 2001, 57, 306–312. [Google Scholar] [CrossRef]
- Zoccola, D.; Moya, A.; Beranger, G.E.; Tambutte, E.; Allemand, D.; Carle, G.F.; Tambutte, S. Specific expression of BMP2/4 ortholog in biomineralizing tissues of corals and action on mouse BMP receptor. Mar. Biotechnol. 2009, 11, 260–269. [Google Scholar] [CrossRef]
- Hayward, D.C.; Samuel, G.; Pontynen, P.C.; Catmull, J.; Saint, R.; Miller, D.J.; Ball, E.E. Localized expression of a dpp/BMP2/4 ortholog in a coral embryo. Proc. Natl. Acad. Sci. USA 2002, 99, 8106–8111. [Google Scholar] [CrossRef]
- Westbroek, P.; Marin, F. A marriage of bone and nacre. Nature 1998, 392, 861–862. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Jiang, P.; Li, J.; Han, F.; Duan, G.; Lu, X.; Gu, Y.; Yu, W. Antibiofilm activity of an exopolysaccharide from marine bacterium Vibrio sp. QY101. PLoS One 2011, 6, e18514. [Google Scholar]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef]
- Shields, R.C.; Mokhtar, N.; Ford, M.; Hall, M.J.; Burgess, J.G.; ElBadawey, M.R.; Jakubovics, N.S. Efficacy of a marine bacterial nuclease against biofilm forming microorganisms isolated from chronic rhinosinusitis. PLoS One 2013, 8, e55339. [Google Scholar]
- Shakir, A.; Elbadawey, M.R.; Shields, R.C.; Jakubovics, N.S.; Burgess, J.G. Removal of biofilms from tracheoesophageal speech valves using a novel marine microbial deoxyribonuclease. Otolaryngol. Head Neck Surg. 2012, 147, 509–514. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef]
- Reddy, S.T.; Chung, K.K.; McDaniel, C.J.; Darouiche, R.O.; Landman, J.; Brennan, A.B. Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: An in vitro study on the effect of sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. J. Endourol. 2011, 25, 1547–1552. [Google Scholar]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Griffith, L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 83–95. [Google Scholar] [CrossRef]
- Pallela, R.; Bojja, S.; Janapala, V.R. Biochemical and biophysical characterization of collagens of marine sponge, Ircinia fusca (Porifera: Demospongiae: Irciniidae). Int. J. Biol. Macromol. 2011, 49, 85–92. [Google Scholar]
- Ehrlich, H. Biological Materials of Marine Origin, 1st ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef]
- Zheng, M.H.; Hinterkeuser, K.; Solomon, K.; Kunert, V.; Pavlos, N.J.; Xu, J. Collagen-derived biomaterials in bone and cartilage repair. Macromol. Symp. 2007, 253, 179–185. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Knieb, C.; Hanke, T. Biomimetically inspired hybrid materials based on silicified collagen. Int. J. Mater. Res. 2007, 98, 603–608. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Addai-Mensah, J.; Losic, D. Silica microcapsules from diatoms as new carrier for delivery of therapeutics. Nanomedicine 2011, 6, 1159–1173. [Google Scholar] [CrossRef]
- Lu, X.; Xia, Y.; Liu, M.; Qian, Y.; Zhou, X.; Gu, N.; Zhang, F. Improved performance of diatomite-based dental nanocomposite ceramics using layer-by-layer assembly. Int. J. Nanomed. 2012, 7, 2153–2164. [Google Scholar]
- Wang, S.; Guo, Z. Bio-inspired encapsulation and functionalization of living cells with artificial shells. Colloids Surf. B Biointerfaces 2014, 113, 483–500. [Google Scholar] [CrossRef]
- Tiffany, M.A. Diatom auxospore scales and early stages in diatom frustule morphogenesis: Their potential for use in nanotechnology. J. Nanosci. Nanotechnol. 2005, 5, 131–139. [Google Scholar] [CrossRef]
- Parkinson, J.; Gordon, R. Beyond micromachining: The potential of diatoms. Trends Biotechnol. 1999, 17, 190–196. [Google Scholar] [CrossRef]
- Gordon, R.; Parkinson, J. Potential roles for diatomists in nanotechnology. J. Nanosci. Nanotechnol. 2005, 5, 35–40. [Google Scholar] [CrossRef]
- Parker, A.R.; Townley, H.E. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2007, 2, 347–353. [Google Scholar] [CrossRef]
- Allison, D.P.; Dufrene, Y.F.; Doktycz, M.J.; Hildebrand, M. Biomineralization at the Nanoscale: Learning from diatoms. Methods Cell Biol. 2008, 90, 61–86. [Google Scholar] [CrossRef]
- Bozarth, A.; Maier, U.G.; Zauner, S. Diatoms in biotechnology: Modern tools and applications. Appl. Microbiol. Biotechnol. 2009, 82, 195–201. [Google Scholar] [CrossRef]
- Bradbury, J. Nature’s nanotechnologists: Unveiling the secrets of diatoms. PLoS Biol. 2004, 2, e306. [Google Scholar] [CrossRef] [Green Version]
- Drum, R.W.; Gordon, R. Star Trek replicators and diatom nanotechnology. Trends Biotechnol. 2003, 21, 325–328. [Google Scholar]
- Almqvist, N.; Delamo, Y.; Smith, B.L.; Thomson, N.H.; Bartholdson, A.; Lal, R.; Brzezinski, M.; Hansma, P.K. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 2001, 202, 518–532. [Google Scholar] [CrossRef]
- Yao, S.; Subhash, G.; Maiti, S. Analysis of nanoindentation response of diatom frustules. J. Nanosci. Nanotechnol. 2007, 7, 4465–4472. [Google Scholar] [CrossRef]
- Subhash, G.; Yao, S.; Bellinger, B.; Gretz, M.R. Investigation of mechanical properties of diatom frustules using nanoindentation. J. Nanosci. Nanotechnol. 2005, 5, 50–56. [Google Scholar] [CrossRef]
- Sen, D.; Buehler, M.J. Structural hierarchies define toughness and defect-tolerance despite simple and mechanically inferior brittle building blocks. Sci. Rep. 2011, 1, 35. [Google Scholar]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning drug loading and release properties of diatom silica microparticles by surface modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef]
- De Stefano, L.; Lamberti, A.; Rotiroti, L.; de Stefano, M. Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter. Acta Biomater. 2008, 4, 126–130. [Google Scholar] [CrossRef]
- Kim, T.W.; Chung, P.W.; Slowing, I.I.; Tsunoda, M.; Yeung, E.S.; Lin, V.S.Y. Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells. Nano Lett. 2008, 8, 3724–3727. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar] [CrossRef]
- Chou, J.; Hao, J.; Hatoyama, H.; Ben-Nissan, B.; Milthorpe, B.; Otsuka, M. The therapeutic effect on bone mineral formation from biomimetic zinc containing tricalcium phosphate (ZnTCP) in zinc-deficient osteoporotic mice. PLoS One 2013, 8, e71821. [Google Scholar]
- Chou, J.H.; Ben-Nissan, B.; Green, D.W.; Valenzuela, S.M.; Kohan, L. Targeting and dissolution characteristics of bone forming and antibacterial drugs by harnessing the structure of microspherical shells from coral beach sand. Adv. Eng. Mater. 2011, 13, 93–99. [Google Scholar] [CrossRef]
- Chou, J.; Ito, T.; Otsuka, M.; Ben-Nissan, B.; Milthorpe, B. The effectiveness of the controlled release of simvastatin from beta-TCP macrosphere in the treatment of OVX mice. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Chou, J.; Valenzuela, S.; Green, D.W.; Kohan, L.; Milthorpe, B.; Otsuka, M.; Ben-Nissan, B. Antibiotic delivery potential of nano- and micro-porous marine structure-derived beta-tricalcium phosphate spheres for medical applications. Nanomedicine (Lond.) 2014. [Google Scholar] [CrossRef]
- Jackson, A.P.; Vincent, J.F.V.; Turner, R.M. The mechanical design of nacre. Proc. R. Soc. Lond. B 1988, 234, 415–440. [Google Scholar] [CrossRef]
- Vincent, J.F.V. Survival of the cheapest. Mater. Today 2002, 5, 28–41. [Google Scholar] [CrossRef]
- Atlan, G.; Balmain, N.; Berland, S.; Vidal, B.; Lopez, E. Reconstruction of human maxillary defects with nacre powder: Histological evidence for bone regeneration. C. R. Acad. Sci. III 1997, 320, 253–258. [Google Scholar] [CrossRef]
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6. [Google Scholar]
- Silve, C.; Lopez, E.; Vidal, B.; Smith, D.C.; Camprasse, S.; Camprasse, G.; Couly, G. Nacre initiates biomineralization by human osteoblasts maintained in vitro. Calcif. Tissue Int. 1992, 51, 363–369. [Google Scholar] [CrossRef]
- Atlan, G.; Delattre, O.; Berland, S.; LeFaou, A.; Nabias, G.; Cot, D.; Lopez, E. Interface between bone and nacre implants in sheep. Biomaterials 1999, 20, 1017–1022. [Google Scholar] [CrossRef]
- Lamghari, M.; Almeida, M.J.; Berland, S.; Huet, H.; Laurent, A.; Milet, C.; Lopez, E. Stimulation of bone marrow cells and bone formation by nacre: In vivo and in vitro studies. Bone 1999, 25, 91S–94S. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Nacre surface transformation to hydroxyapatite in a phosphate buffer solution. Biomaterials 2003, 24, 4323–4331. [Google Scholar]
- Kim, H.; Lee, K.; Ko, C.Y.; Kim, H.S.; Shin, H.I.; Kim, T.; Lee, S.H.; Jeong, D. The role of nacreous factors in preventing osteoporotic bone loss through both osteoblast activation and osteoclast inactivation. Biomaterials 2012, 33, 7489–7496. [Google Scholar] [CrossRef]
- Rousseau, M.; Pereira-Mouries, L.; Almeida, M.J.; Milet, C.; Lopez, E. The water-soluble matrix fraction from the nacre of Pinctada maxima produces earlier mineralization of MC3T3-E1 mouse pre-osteoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 1–7. [Google Scholar]
- Bedouet, L.; Rusconi, F.; Rousseau, M.; Duplat, D.; Marie, A.; Dubost, L.; Le Ny, K.; Berland, S.; Peduzzi, J.; Lopez, E. Identification of low molecular weight molecules as new components of the nacre organic matrix. Comp. Biochem. Phys. B 2006, 144, 532–543. [Google Scholar] [CrossRef]
- Doherty, M.J.; Schlag, G.; Schwarz, N.; Mollan, R.A.; Nolan, P.C.; Wilson, D.J. Biocompatibility of xenogeneic bone, commercially available coral, a bioceramic and tissue sealant for human osteoblasts. Biomaterials 1994, 15, 601–608. [Google Scholar] [CrossRef]
- Soost, F. Biocoral—An alternative bone substitute. Chirurg 1996, 67, 1193–1196. [Google Scholar] [CrossRef]
- White, E.; Shors, E.C. Biomaterial aspects of Interpore-200 porous hydroxyapatite. Dent. Clin. North Am. 1986, 30, 49–67. [Google Scholar]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef]
- Swider, P.; Conroy, M.; Pedrono, A.; Ambard, D.; Mantell, S.; Soballe, K.; Bechtold, J.E. Use of high-resolution MRI for investigation of fluid flow and global permeability in a material with interconnected porosity. J. Biomech. 2007, 40, 2112–2118. [Google Scholar] [CrossRef]
- White, E.W.; Weber, J.N.; Roy, D.M.; Owen, E.L.; Chiroff, R.T.; White, R.A. Replamineform porous biomaterials for hard tissue implant applications. J. Biomed. Mater. Res. 1975, 9, 23–27. [Google Scholar] [CrossRef]
- Souyris, F.; Pellequer, C.; Payrot, C.; Servera, C. Coral, a new biomedical material. Experimental and first clinical investigations on Madreporaria. J. Maxillofac. Surg. 1985, 13, 64–69. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lee, T.M.; Chiu, K.H.; Shaw, S.Y.; Yang, C.Y. A comparative study of the physical and mechanical properties of three natural corals based on the criteria for bone-tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 1273–1280. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Milev, A.; Vago, R. Morphology of sol-gel derived nano-coated coralline hydroxyapatite. Biomaterials 2004, 25, 4971–4975. [Google Scholar] [CrossRef]
- Zreiqat, H.; Valenzuela, S.M.; Nissan, B.B.; Roest, R.; Knabe, C.; Radlanski, R.J.; Renz, H.; Evans, P.J. The effect of surface chemistry modification of titanium alloy on signaling pathways in human osteoblasts. Biomaterials 2005, 26, 7579–7586. [Google Scholar] [CrossRef]
- Dunlop, J.W.C.; Weinkamer, R.; Fratzl, P. Artful interfaces within biological materials. Mater. Today 2011, 14, 70–78. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Green, D.W.; Lai, W.-F.; Jung, H.-S. Evolving Marine Biomimetics for Regenerative Dentistry. Mar. Drugs 2014, 12, 2877-2912. https://doi.org/10.3390/md12052877
Green DW, Lai W-F, Jung H-S. Evolving Marine Biomimetics for Regenerative Dentistry. Marine Drugs. 2014; 12(5):2877-2912. https://doi.org/10.3390/md12052877
Chicago/Turabian StyleGreen, David W., Wing-Fu Lai, and Han-Sung Jung. 2014. "Evolving Marine Biomimetics for Regenerative Dentistry" Marine Drugs 12, no. 5: 2877-2912. https://doi.org/10.3390/md12052877
APA StyleGreen, D. W., Lai, W.-F., & Jung, H.-S. (2014). Evolving Marine Biomimetics for Regenerative Dentistry. Marine Drugs, 12(5), 2877-2912. https://doi.org/10.3390/md12052877





