The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives
Abstract
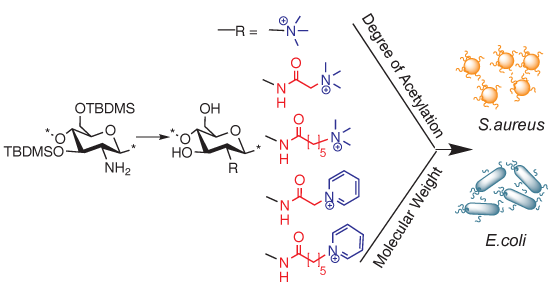
:1. Introduction
2. Results and Discussion
2.1. Synthesis of N-(2-(N,N,N-Trimethylammoniumyl)acetyl)-chitosan Chloride (TMA-CS) and N-(2-(1-Pyridiniumyl)acetyl)-chitosan Chloride (PyA-CS), the C-2 Spacer Chitosan Derivatives




2.2. Synthesis of N-(6-(N,N,N-Trimethylammoniumyl)hexanoyl)-chitosan Chloride (TMHA-CS) and N-(6-(1-Pyridiniumyl)hexanoyl)-chitosan Chloride (PyHA-CS), C-6 Spacer Derivatives



2.3. Synthesis of C-0 Spacer TMC Derivatives (15i–v)

2.4. Physicochemical Properties of Chitosan-Derivatives
| Parent Chitosan Material | DA (%) | Chitosan (1i–v) | Mes-CS (2i–v) | TMA-CS (6i–v) | PyA-CS (8i–v) | TMHA-CS (11i–v) | PyHA-CS (13i–v) | TMC (15i–v) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw | (PDI) | Mw | (PDI) | Mw | (PDI) | Mw | (PDI) | Mw | (PDI) | Mw | (PDI) | Mw | (PDI) | ||
| CS-i | 7 | 235 | (2.8) | 24.6 | (1.6) | 23.8 | (2.1) | 18.8 | (1.8) | 17.3 | (1.6) | 12.9 | (1.5) | 18.7 | (1.5) |
| CS-ii | 6 | 294 | (2.3) | 20.8 | (1.9) | 17.1 | (1.8) | 17.3 | (2.0) | 15.1 | (1.5) | 9.8 | (1.3) | 15.3 | (1.4) |
| CS-iii | 17.3 | 225 | (2.6) | 19.1 | (1.6) | 16.4 | (1.6) | 12.2 | (1.9) | 13.1 | (1.4) | - | - | 13.2 | (1.4) |
| CS-iv | 19 | 308 | (2.6) | 21.4 | (2.4) | 16.7 | (1.6) | 14.6 | (1.8) | 14.2 | (1.8) | 15.8 | (1.1) | 19.8 | (1.6) |
| CS-v | 34.2 | 180 | (2.9) | 19.5 | (1.5) | 18.9 | (1.7) | 10.8 | (1.4) | 7.4 | (1.4) | - | - | 16.5 | (1.5) |
2.5. Antibacterial Properties
| Compounds | Structure | S. aureus (ATCC 29213) | E. coli (ATCC 25922) | HC50 (μg/mL) | Selectivity (HC50/MIC) | EC50 (μg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MLC (μg/mL) | MIC (μg/mL) | MLC (μg/mL) | S. aureus | E. coli | ||||
| TMC (15i) |  | 8 | 64 | 256 | 256 | 6114 | 764 | 47.7 | 40 |
| TMC (15ii) | 32 | 32 | 64 | 64 | 6114 | 191 | 95.5 | - | |
| TMC (15iii) | 4 | 4 | 64 | 64 | 6114 | 1528 | 95.5 | - | |
| TMC (15iv) | 8 | 8 | 256 | 256 | 3072 | 764 | 47.7 | 10 | |
| TMC (15v) | 32 | 32 | 256 | 1024 | 640 | 191 | - | - | |
| TMA-CS (6i) |  | 8 | 8 | 16,384 | ≥32,768 | ≥32,768 | ≥4096 | ≥2 | 26 |
| TMA-CS (6ii) | 8 | 8 | 16,384 | 16,384 | ≥32,768 | ≥4096 | ≥2 | - | |
| TMA-CS (6iii) | 32 | 32 | 16,384 | 16,384 | ≥32,768 | ≥1024 | ≥2 | - | |
| TMA-CS (6iv) | 32 | 32 | ≥32,768 | ≥32,768 | ≥32,768 | ≥1024 | - | 66 | |
| TMA-CS (6v) | 128 | 128 | ≥32,768 | ≥32,768 | ≥32,768 | ≥256 | - | - | |
| PyA-CS (8i) |  | 8 | 1024 | 16,384 | 16,384 | ≥32,768 | ≥8192 | ≥2 | 38 |
| PyA-CS (8ii) | 8 | 512 | 8192 | 8192 | ≥32,768 | ≥8192 | ≥4 | - | |
| PyA-CS (8iii) | 1024 | 1024 | 16,384 | 16,384 | ≥32,768 | ≥8 | ≥2 | - | |
| PyA-CS (8iv) | 512 | 1024 | 16,384 | 16,384 | ≥32,768 | ≥16 | ≥2 | 12 | |
| PyA-CS (8v) | 512 | 512 | 128 | 8192 | ≥32,768 | ≥64 | ≥256 | - | |
| TMHA-CS (11i) |  | 1024 | 2048 | 256 | ≥32,768 | ≥32,768 | ≥32 | ≥128 | 644 |
| TMHA-CS (11ii) | 2048 | 2048 | 512 | 16,384 | ≥32,768 | ≥16 | ≥64 | - | |
| TMHA-CS (11iii) | 1024 | 2048 | 128 | ≥32,768 | ≥32,768 | ≥4 | ≥256 | - | |
| TMHA-CS (11iv) | 2048 | 4096 | 512 | ≥32,768 | ≥32,768 | ≥8 | ≥64 | 108 | |
| TMHA-CS (11v) | 1024 | 4096 | 1024 | ≥32,768 | ≥32,768 | ≥32 | ≥32 | - | |
| PyHA-CS (13i) |  | 4096 | 4096 | ≥32,768 | ≥32,768 | ≥32,768 | ≥8 | - | 4 |
| PyHA-CS (13ii) | 2048 | 2048 | ≥32,768 | ≥32,768 | ≥32,768 | ≥32 | - | - | |
| PyHA-CS (13iii) | 8192 | 8192 | ≥32,768 | ≥32,768 | ≥32,768 | ≥4 | - | - | |
| PyHA-CS (13iv) | 2048 | 2048 | 16,384 | 16,384 | ≥32,768 | ≥16 | ≥2 | 18 | |
| PyHA-CS (13v) | 2048 | 2048 | ≥32,768 | ≥32,768 | ≥32,768 | ≥16 | - | - | |

2.6. Hemolytic Activity and Cytotoxicity

3. Experimental Section
3.1. Materials
3.2. Characterization and Calculations

3.3. Gel Permeation Chromatography (GPC)
3.4. Chemical Synthesis
3.4.1. General Procedure for N-Quaternized-acetyl-chitosan Derivatives
3.4.2. General Procedure for N-Quaternized-hexanoyl-chitosan Derivatives
3.4.3. General TBDMS Deprotection Procedure to Give the Final Quaternary Ammoniumyl and Pyridiniumyl Derivatives (6i–v, 8i–v, 11i–v, 13i–v)
3.4.4. General Procedure for N-Quaternized-chitosan
3.5. Biological Methods
3.5.1. Bacterial Strains, Media and Culture Conditions
3.5.2. Hemolytic Activity

3.5.3. Cytotoxicity
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, X.; Li, L.; Zhou, J.; Lu, S.; Yang, J.; Yin, X.; Ren, J. Preparation and characterization of N-succinyl-N′-octyl chitosan micelles as doxorubicin carriers for effective anti-tumor activity. Colloids Surf. B Biointerfaces 2007, 55, 222–228. [Google Scholar] [CrossRef]
- Sharon, S.; Katanchalee, M.-N. Chitosan based surfactant polymers designed to improve blood compatibility on biomaterials. Colloids Surf. B Biointerfaces 2005, 42, 147–155. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar]
- De Britto, D.; de Assis, O.B.G. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 2007, 41, 198–203. [Google Scholar] [CrossRef]
- Rivero, S.G.M.; Garcia, M.A.; Pinotti, A.N. Biodegradable Film, Process for Its Preparation and Uses. Patent AR 80,876A1, 16 May 2012. [Google Scholar]
- Huang, D.; Huang, Y.; Xu, F.; Li, X.; Zhao, Y. Degradable Non-Toxic Seed Dressing Formulation Containing Chitosan, Polyvinyl Alcohol and Plant Extract, and Preparation Method Thereof. Patent CN 101,416,650A, 29 April 2009. [Google Scholar]
- Aiedeh, K.; Taha, M.O. Synthesis of iron-crosslinked chitosan succinate and iron-crosslinked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release beads. Eur. J. Pharm. Sci. 2001, 13, 159–168. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Chitosan and Its Derivatives for Drug Delivery Perspective. Adv. Polym. Sci. 2011, 243, 23–54. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Avadi, M.R.; Zohuriaan-Mehr, M.J.; Younessi, P.; Amini, M.; Tehrani, M.R.; Shafiee, A. Optimized synthesis and characterization of N-triethyl chitosan. J. Bioact. Compat. Polym. 2003, 18, 469–479. [Google Scholar] [CrossRef]
- Seong, H.-S.; Whang, H.S.; Ko, S.-W. Synthesis of a quaternary ammonium derivative of chito-oligosaccharide as antimicrobial agent for cellulosic fibers. J. Appl. Polym. Sci. 2000, 76, 2009–2015. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022. [Google Scholar] [CrossRef]
- Benediktsdóttir, B.E.; Baldursson, Ó.; Másson, M. Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application. J. Control. Release 2014, 173, 18–31. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Soininen, P.; Elomaa, M.; Safin, R.; Masson, M.; Jarvinen, T. N-chloroacyl-6-O-triphenylmethylchitosans: Useful intermediates for synthetic modifications of chitosan. Biomacromolecules 2005, 6, 858–863. [Google Scholar]
- Holappa, J.; Nevalainen, T.; Soininen, P.; Masson, M.; Jarvinen, T. Synthesis of novel quaternary chitosan derivatives via N-chloroacyl-6-O-triphenylmethylchitosans. Biomacromolecules 2006, 7, 407–410. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Savolainen, J.; Soininen, P.; Elomaa, M.; Safin, R.; Suvanto, S.; Pakkanen, T.; Masson, M.; Loftsson, T.; et al. Synthesis and characterization of chitosan N-betainates having various degrees of substitution. Macromolecules 2004, 37, 2784–2789. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Safin, R.; Soininen, P.; Asplund, T.; Luttikhedde, T.; Masson, M.; Jarvinen, T. Novel water-soluble quaternary piperazine derivatives of chitosan: Synthesis and characterization. Macromol. Biosci. 2006, 6, 139–144. [Google Scholar] [CrossRef]
- Masson, M.; Holappa, J.; Hjalmarsdottir, M.; Runarsson, O.V.; Nevalainen, T.; Jarvinen, T. Antimicrobial activity of piperazine derivatives of chitosan. Carbohydr. Polym. 2008, 74, 566–571. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Song, W.L.; Gaware, V.S.; Runarsson, O.V.; Masson, M.; Mano, J.F. Functionalized superhydrophobic biomimetic chitosan-based films. Carbohydr. Polym. 2010, 81, 140–144. [Google Scholar] [CrossRef]
- Runarsson, O.V.; Malainer, C.; Holappa, J.; Sigurdsson, S.T.; Masson, M. tert-Butyldimethylsilyl O-protected chitosan and chitooligosaccharides: Useful precursors for N-modifications in common organic solvents. Carbohydr. Res. 2008, 343, 2576–2582. [Google Scholar]
- Gaware, V.S.; Hakerud, M.; Leosson, K.; Jonsdottir, S.; Hogset, A.; Berg, K.; Masson, M. Tetraphenylporphyrin tethered chitosan based carriers for photochemical transfection. J. Med. Chem. 2013, 56, 807–819. [Google Scholar] [CrossRef]
- Benediktsdottir, B.E.; Gaware, V.S.; Runarsson, O.V.; Jonsdottir, S.; Jensen, K.J.; Masson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B: Biointerfaces 2008, 65, 197–202. [Google Scholar]
- Je, J.-Y.; Kim, S.K. Antimicrobial action of novel chitin derivative. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 104–109. [Google Scholar]
- Je, J.Y.; Kim, S.K. Chitosan Derivatives Killed Bacteria by Disrupting the Outer and Inner Membrane. J. Agric. Food Chem. 2006, 54, 6629–6633. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Rurián-Henares, J.A.; Morales, F.J. Antimicrobial Activity of Melanoidins against Escherichia coli Is Mediated by a Membrane-Damage Mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef]
- Sajomsang, W.; Ruktanonchai, U.R.; Gonil, P.; Warin, C. Quaternization of N-(3-pyridylmethyl) chitosan derivatives: Effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N,N,N-trimethyl ammonium moieties on bactericidal activity. Carbohydr. Polym. 2010, 82, 1143–1152. [Google Scholar] [CrossRef]
- Jing, Y.J.; Hao, Y.J.; Qu, H.; Shan, Y.; Li, D.S.; Du, R.Q. Studies on the antibacterial activities and mechanisms of chitosan obtained from cuticles of housefly larvae. Acta Biol. Hung. 2007, 58, 75–86. [Google Scholar]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.-B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar]
- Chung, Y.C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Byun, H.G.; Moon, S.H.; Kim, S.-K. Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J. Microbiol. Biotechnol. 2004, 14, 317–323. [Google Scholar]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 18, 734–736. [Google Scholar] [CrossRef]
- Stirling, C.J.M. Intramolecular reactions of amides. Part II. Cyclisation of amides of [small omega]-bromo-carboxylic, acids. J. Chem. Soc. (Resumed) 1960, 49, 255–262. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar]
- Runarsson, O.V.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjalmarsdottir, M.; Nevalainen, T.; Masson, M. Antibacterial activity of N-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (SAR) investigations. Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- Holappa, J.; Hjalmarsdottir, M.; Masson, M.; Runarsson, O.; Asplund, T.; Soininen, P.; Nevalainen, T.; Jarvinen, T. Antimicrobial activity of chitosan N-betainates. Carbohydr. Polym. 2006, 65, 114–118. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Lalitha, R.G.; Tharanathan, R.N. Low molecular weight chitosans: Preparation with the aid of papain and characterization. Biochim. Biophys. Acta Gen. Subj. 2004, 1670, 137–146. [Google Scholar]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar]
- Pavinatto, A.; Pavinatto, F.J.; Barros-Timmons, A.; Oliveira, O.N. Electrostatic Interactions Are Not Sufficient to Account for Chitosan Bioactivity. ACS Appl. Mater. Interfaces 2009, 2, 246–251. [Google Scholar]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar]
- Lienkamp, K.; Madkour, A.; Tew, G. Antibacterial Peptidomimetics: Polymeric Synthetic Mimics of Antimicrobial Peptides. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens; Abe, A., Kausch, H.H., Möller, M., Pasch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 251, pp. 141–172. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 8th ed.; Approved Standard. CLSI document M07-A8; Clinical Laboratory Standards Instiute: Wayne, PA, USA, 2009; Volume 29. [Google Scholar]
- Ilker, M.F.; Nusslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar]
- Zhou, C.; Qi, X.; Li, P.; Chen, W.N.; Mouad, L.; Chang, M.W.; Leong, S.S.; Chan-Park, M.B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of alpha-aminoacid-N-carboxyanhydrides. Biomacromolecules 2010, 11, 60–67. [Google Scholar]
- Liang, Z.; Zhu, M.; Yang, Y.W.; Gao, H. Antimicrobial activities of polymeric quaternary ammonium salts from poly(glycidyl methacrylate)s. Polym. Adv. Technol. 2014, 25, 117–122. [Google Scholar] [CrossRef]
- Yalinca, Z.; Yilmaz, E.; Taneri, B.; Bullici, F.T. A comparative study on antibacterial activities of chitosan based products and their combinations with gentamicin against S. epidermidis and E. coli. Polym. Bull. 2013, 70, 3407–3423. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Sabaa, M.W.; El-Ghandour, A.H.; Abdel-Aziz, M.M.; Abdel-Gawad, O.F. Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int. J. Biol. Macromol. 2013, 60, 156–164. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Mar. Drugs 2014, 12, 4635-4658. https://doi.org/10.3390/md12084635
Sahariah P, Gaware VS, Lieder R, Jónsdóttir S, Hjálmarsdóttir MÁ, Sigurjonsson OE, Másson M. The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Marine Drugs. 2014; 12(8):4635-4658. https://doi.org/10.3390/md12084635
Chicago/Turabian StyleSahariah, Priyanka, Vivek S. Gaware, Ramona Lieder, Sigríður Jónsdóttir, Martha Á. Hjálmarsdóttir, Olafur E. Sigurjonsson, and Már Másson. 2014. "The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives" Marine Drugs 12, no. 8: 4635-4658. https://doi.org/10.3390/md12084635
APA StyleSahariah, P., Gaware, V. S., Lieder, R., Jónsdóttir, S., Hjálmarsdóttir, M. Á., Sigurjonsson, O. E., & Másson, M. (2014). The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Marine Drugs, 12(8), 4635-4658. https://doi.org/10.3390/md12084635