Characterization of an Alginate Lyase, FlAlyA, from Flavobacterium sp. Strain UMI-01 and Its Expression in Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Characterization of Strain UMI-01
2.2. Purification of FlAlyA


| Purification Steps | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Crude enzyme | 1500.0 | 102,000 | 68 | 100.0 | 1.0 |
| AS fractionation | 61.0 | 29,646 | 486 | 29.3 | 7.2 |
| DEAE-650M | 0.8 | 9849 | 12,627 | 9.9 | 186.8 |
| Purified FlAlyA | 0.3 | 6574 | 23,478 | 6.5 | 347.3 |
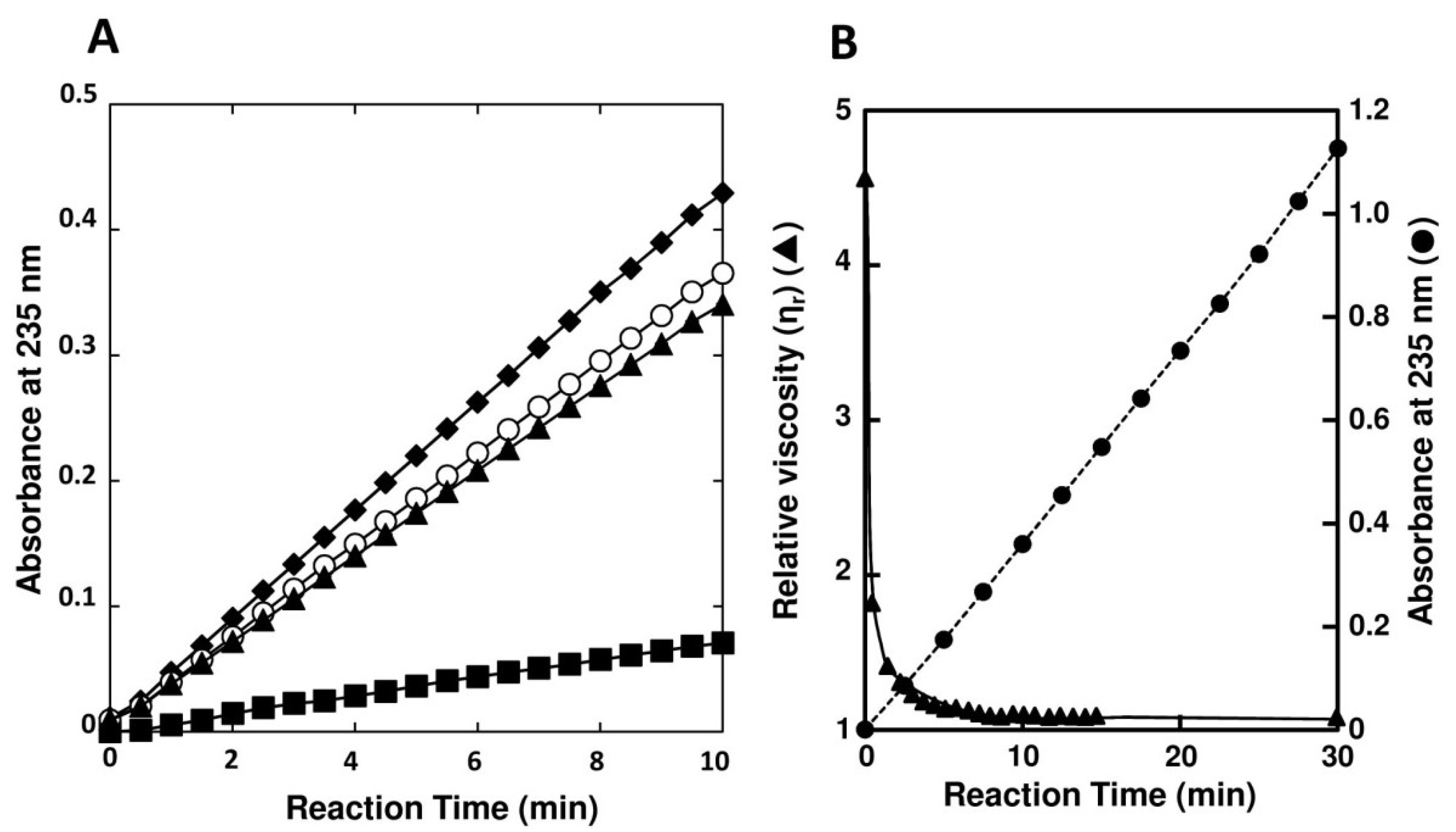
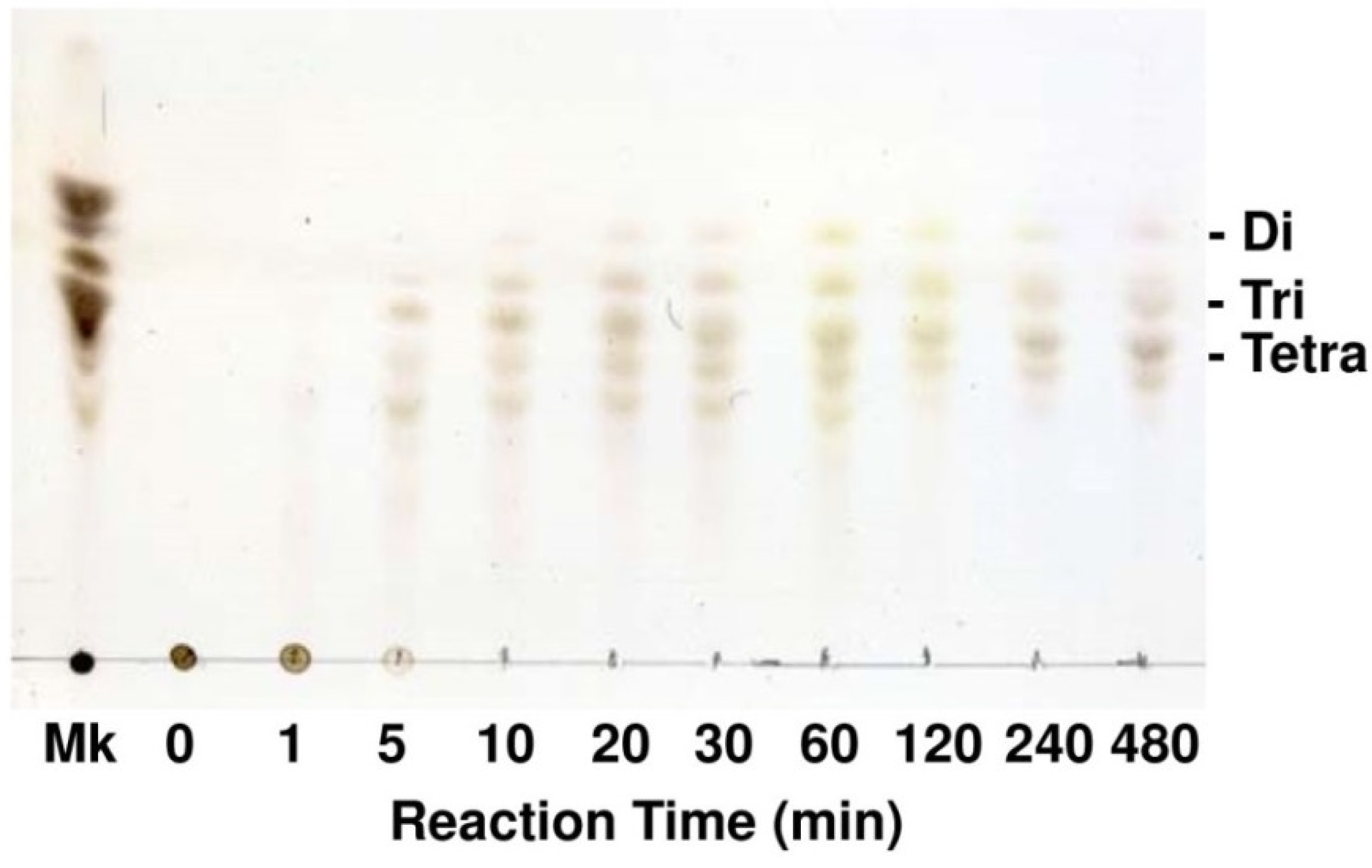
2.3. Enzymatic Properties of FlAlyA
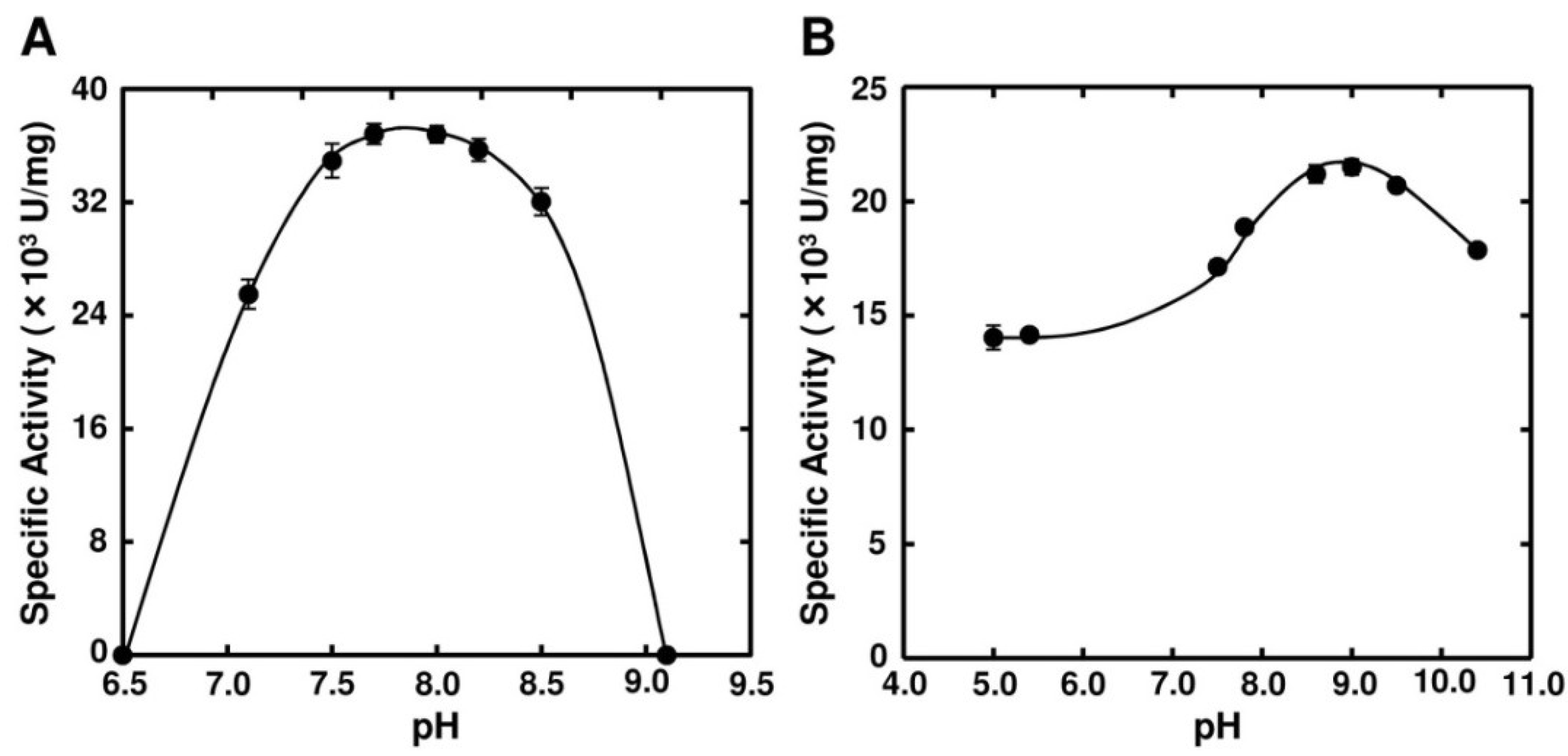
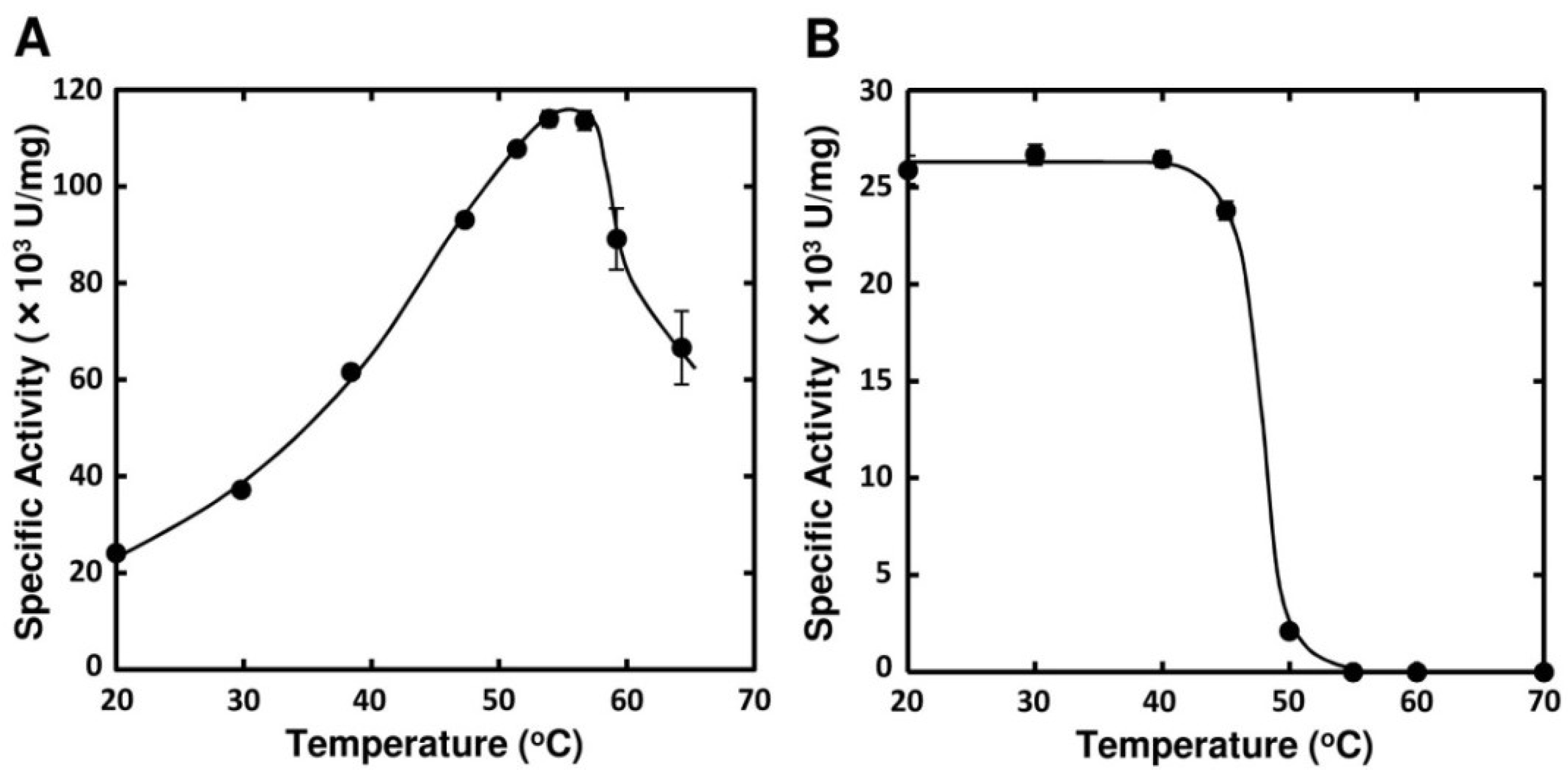
| Substances | Concentration (mM) | Relative Activity (%) | ||
|---|---|---|---|---|
| Non | 100.0 | ± | 2.0 | |
| NaCl | 100 | 134.1 | ± | 1.0 |
| NaCl | 5 | 106.6 | ± | 0.5 |
| KCl | 5 | 108.8 | ± | 1.0 |
| CaCl2 | 5 | 118.8 | ± | 0.6 |
| MgCl2 | 5 | 126.0 | ± | 0.9 |
| CoCl2 | 5 | 57.1 | ± | 2.3 |
| MnCl2 | 5 | 101.1 | ± | 0.7 |
| NiCl2 | 5 | 47.1 | ± | 1.0 |
| EDTA | 5 | 95.4 | ± | 1.1 |
| EGTA | 5 | 106.4 | ± | 0.9 |
| Dithiothreitol | 5 | 93.2 | ± | 0.6 |
| 2-Mercaptoethanol | 5 | 92.6 | ± | 0.5 |
2.4. Analysis for Primary Structure of FlAlyA
| Primer Name | Nucleotide Sequence (Corresponding Amino-Acid Sequence) |
|---|---|
| FlAly-1F | 5′-WSNRACNGCNAARATHGAYTGG-3′ |
| (SKTAKIDW) | |
| FlAly-2F | 5′-TGGWSNCAYTGGACNGTNACNGTNCC-3′ |
| (WSHWTVTVP) | |
| PL7-Rv | 5′-TANARNCCNGCYTTRAARTA-3′ |
| (YFKAGNY) | |
| InvFw1 | 5′-AATGAGAGGTACGTATGCTATTGACGAC-3′ |
| InvFw2 | 5′-GCGTTATTATTGCGCAAATTCACGG-3′ |
| InvRv1 | 5′-CAACAGACTTGTCTTTTGGGTCATCGTA-3′ |
| InvRv2 | 5′-GGATGCGATTTTATCCTCAGCATAATTT-3′ |
| FlAlyA-Fw | 5′-ACCAAAGTGGTAGAATAATAAAA-3′ |
| FlAlyA-Rv | 5′-ACACTTTAAAACAGATTAGCTAAACCG-3′ |
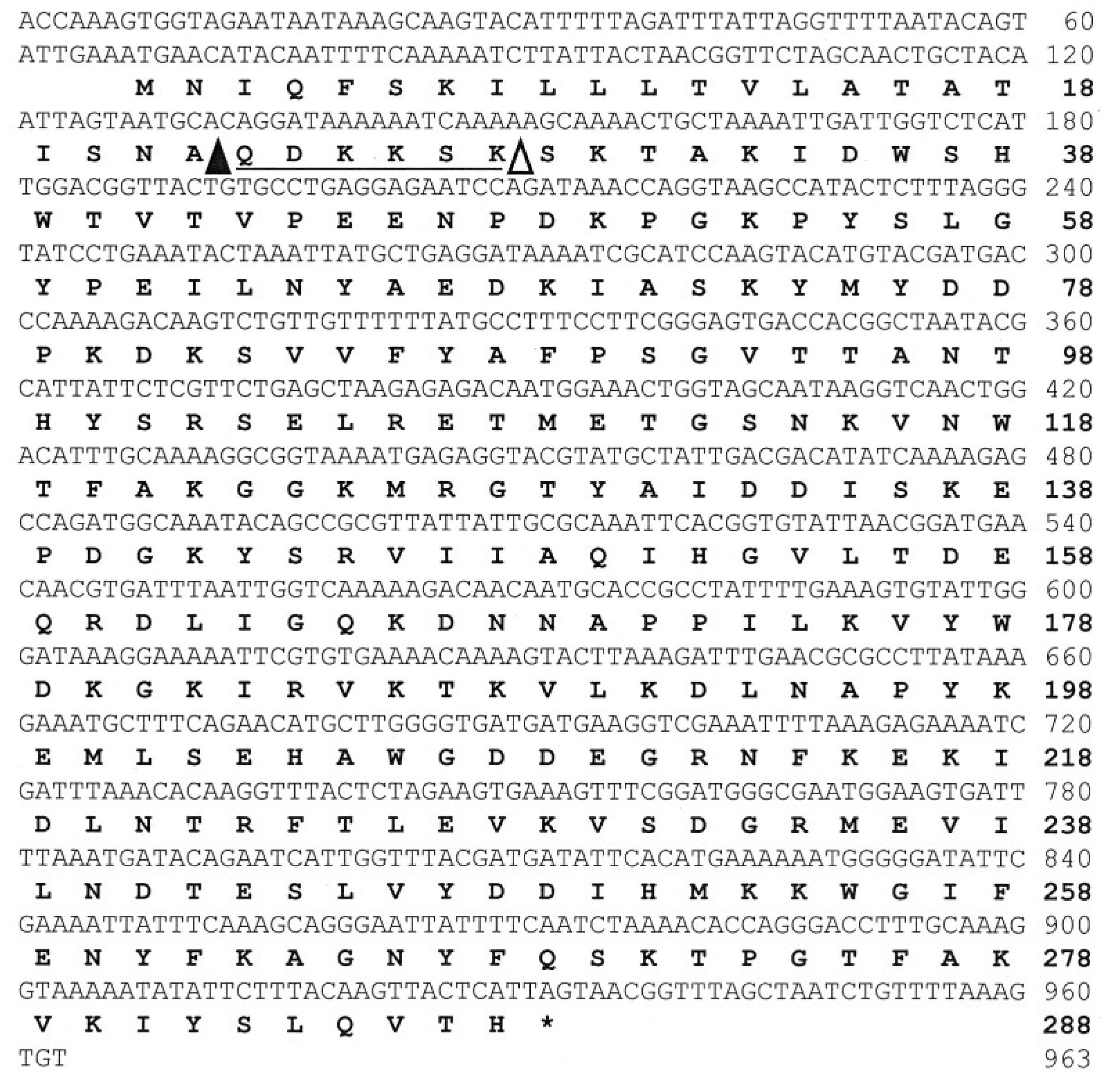
2.5. Expression of Recombinant FlAlyA in E. coli
| Fractions | Total Protein (mg) | Total Activity (U) | Activity Yield (%) |
|---|---|---|---|
| Periplasmic | 12.35 | 4688 | 5.6 |
| Cytosolic | 75.45 | 79,584 | 94.4 |
| Sum of fractions | 84,272 | 100.0 |
3. Discussion

4. Experimental Section
4.1. Materials
4.2. Isolation of Strain UMI-01
4.3. Preparation of Periplasmic Fraction from Strain UMI-01
4.4. Assay for Alginate Lyase Activity
4.5. Thin Layer Chromatography for Degradation Products of Alginate
4.6. SDS-PAGE and Zymography
4.7. Determination of Protein Concentration
4.8. Determination of N-Terminal Amino-Acid Sequence
4.9. Cloning and Sequencing of FlAlyA Gene
4.10. Expression of Recombinant FlAlyA in Escherichia coli
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haug, A.; Larsen, B.; Smidsrod, O. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Gacesa, P. Enzymatic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [PubMed]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Coutinho, P.M.; Levasseur, A.; Lombard, V.; Drula, E. CAZY Carbohydrate-Active enZYmes Database. Available online: http://www.cazy.org (accessed on 15 April 2014).
- Tomoda, Y.; Umemura, K.; Adachi, T. Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate. Biosci. Biotechnol. Biochem. 1994, 58, 202–203. [Google Scholar] [CrossRef]
- Sutherland, I.W. Polysaccharide lyases. FEMS Microbiol. Rev. 1995, 16, 323–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomericuronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Endo, T.; Nakakita, R.; Murata, K.; Yonemoto, Y.; Okayama, K. Effect of depolymerized alginates on the growth of Bifidobacteria. Biosci. Biotechnol. Biochem. 1992, 56, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Ariyo, B.; Tamerler, C.; Bucke, C.; Keshavarz, T. Enhanced penicillin production by oligosaccharides from batch culture of Penicillium chrysogenum in stirred-tank reactors. FEMS Microbiol. Lett. 1998, 166, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine production in human mononuclear cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Tajima, S.; Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999, 291, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Stimulation of multiple cytokine production in mice by alginate oligosaccharides following intraperitoneal administration. Carbohydr. Res. 2007, 342, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Chaki, T.; Ogawa, H. Effect of sodium alginate oligosaccharide on human blood pressure. Med. Cons. New-Remed. 2001, 38, 555–560. (In Japanese) [Google Scholar]
- Chaki, T.; Nishimoto, S.; Hiura, N.; Satou, R.; Tou, Y.; Kakinuma, S. Effect of a powdered drink containing sodium alginate oligosaccharide on blood pressure in mild hypertensive and high normal blood pressure subjects. J. Nutr. Food 2002, 5, 41–54. (In Japanese) [Google Scholar]
- Takeda, H.; Yoneyama, F.; Kawai, S.; Hashimoto, W.; Murata, K. Bioethanol production from marine biomass alginate by metabolically engineered bacteria. Energy Environ. Sci. 2011, 4, 2575–2581. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo1, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman1, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brownmacroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar]
- Murata, K.; Inose, T.; Hisano, T.; Abe, S.; Yonemoto, Y.; Yamashita, T.; Takagi, M.; Sakaguchi, K.; Kumura, A.; Imanaka, T. Bacterial alginate lyase: Enzymology, genetics and application. J. Ferment. Bioeng. 1993, 76, 427–437. [Google Scholar] [CrossRef]
- Sawabe, T.; Ohtsuka, M.; Ezura, Y. Novel alginate lyases from marine bacterium Alteromonas sp. strain H-4. Carbohydr. Res. 1997, 28, 69–76. [Google Scholar]
- Zhang, Z.; Yu, G.; Guan, H.; Zhao, X.; Du, Y.; Jiang, X. Preparation and structural elucidation of alginate oligosaccharides degraded by alginate lyase from Vibrio sp. 510. Carbhydr. Res. 2004, 339, 1475–1481. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J.; Li, X.; Peng, Q.; Lu, H.; Du, Y. Characterization of a new alginate lyase from newly isolated Flavobacterium sp. S20. J. Ind. Microbiol. Biotechnol. 2012, 40, 113–122. [Google Scholar] [CrossRef]
- Shimizu, E.; Ojima, T.; Nishita, K. cDNA cloning of an alginate lyase from abalone, Haliotis discus hannai. Carbohydr. Res. 2003, 338, 2841–2852. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Suzuki, K.; Inoue, A.; Ojima, T. A novel oligoalginate lyase from abalone, Haliotis discus hannai, that releases disaccharide from alginate polymer in an exolytic manner. Carbohydr. Res. 2006, 341, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Inoue, A.; Tanaka, H.; Ojima, T. Isolation and characterization of two alginate lyase isozymes, AkAly28 and AkAly33, from the common sea hare Aplysia kurodai. Comp. Biochem. Physiol. B 2010, 157, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Inoue, A.; Tanaka, H.; Ojima, T. cDNA cloning of an alginate lyase from a marine gastropod Aplysia kurodai and assessment of catalytically important residues of this enzyme. Biochimie 2011, 93, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rahman, M.M.; Inoue, A.; Ojima, T. Heat-stability and primary structure of the major alginate lyase isozyme LbAly35 from Littorina brevicula. Fisheries Sci. 2012, 78, 889–896. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, L.; Inoue, A.; Ojima, T. cDNA cloning and bacterial expression of a PL-14 alginate lyase from a herbivorous marine snail Littorina brevicula. Carbohydr. Res. 2012, 360, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Brunak, S. SignalP 4.0 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 10 March 2014).
- Mai, K.; Mercer, J.P.; Donlon, J. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. III. Response of abalone to various levels of dietary lipid. Aquaculture 1995, 134, 65–80. [Google Scholar]
- Takami, H.; Kawamura, T.; Yamashita, Y. Development of polysaccharide degradation activity in postlarval abalone Haliotis discus hannai. J. Shellfish Res. 1998, 17, 723–727. [Google Scholar]
- Johnston, D.; Moltschaniwskyj, N.; Wells, J. Development of the radula and digestive system of juvenile blacklip abalone (Haliotis rubra): Potential factors responsible for variable weaning success on artificial diets. Aquaculture 2005, 250, 341–355. [Google Scholar] [CrossRef]
- Lewin, R.A. Algal Physiology and Biochemistry, Biochemical Taxonomy in Botanical Monographs; Stewart, W.D.P., Ed.; Blackwell Publishing: Oxford/London, UK, 1974; Volume 10, pp. 1–32. [Google Scholar]
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses; Chapman and Hall publishing Ltd.: New York, NY, USA, 1980; pp. 30–240. [Google Scholar]
- Hashimoto, W.; Miyake, O.; Momma, K.; Kawai, S.; Murata, K. Molecular identification of oligoalginate lyase of Sphingomonas sp. strain A1 as one of the enzymes required for complete depolymerization of alginate. J. Bacteriol. 2000, 182, 4572–4577. [Google Scholar]
- Yamasaki, M.; Ogura, K.; Hashimoto, W.; Mikami, B.; Murata, K. A structural basis for depolymerization of alginate by polysaccharide lyase family-7. J. Mol. Biol. 2005, 352, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Yamasaki, M.; Mikami, B.; Hashimoto, W.; Murata, K. Substrate recognition by family 7 alginate lyase from Sphingomonas sp. A1. J. Mol. Biol. 2008, 380, 373–385. [Google Scholar] [CrossRef]
- Thomas, F.; Lundqvist, L.C.E.; Jam, M.; Jeudy, A.; Barbeyron, T.; Sandström, C.; Michel, G.; Czjzek, M. Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J. Biol. Chem. 2013, 288, 23021–23037. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Moriwaki, S.; Miyake, O.; Hashimoto, W.; Murata, K.; Mikami, B. Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7: A novel alginate lyase with a beta-sandwich fold. J. Biol. Chem. 2004, 279, 31863–31872. [Google Scholar] [CrossRef] [PubMed]
- Gasesa, P.; Wusteman, F.S. Plate assay for simultaneous detection of alginate lyases and determination of substrate specificity. Appl. Environ. Microbiol. 1990, 56, 2265–2267. [Google Scholar] [PubMed]
- Morris, E.R.; Rees, D.A.; Thom, D. Characterisation of alginate composition and block-structure by circular dichroism. Carbohydr. Res. 1980, 81, 305–314. [Google Scholar] [CrossRef]
- Quan, S.; Hiniker, A.; Collet, J.F.; Bardwell, C.A. Isolation of bacteria envelope proteins. Methods Mol. Biol. 2013, 966, 359–366. [Google Scholar] [PubMed]
- Porzio, M.A.; Pearson, A.M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim. Biophys. Acta 1977, 490, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inoue, A.; Takadono, K.; Nishiyama, R.; Tajima, K.; Kobayashi, T.; Ojima, T. Characterization of an Alginate Lyase, FlAlyA, from Flavobacterium sp. Strain UMI-01 and Its Expression in Escherichia coli. Mar. Drugs 2014, 12, 4693-4712. https://doi.org/10.3390/md12084693
Inoue A, Takadono K, Nishiyama R, Tajima K, Kobayashi T, Ojima T. Characterization of an Alginate Lyase, FlAlyA, from Flavobacterium sp. Strain UMI-01 and Its Expression in Escherichia coli. Marine Drugs. 2014; 12(8):4693-4712. https://doi.org/10.3390/md12084693
Chicago/Turabian StyleInoue, Akira, Kohei Takadono, Ryuji Nishiyama, Kenji Tajima, Takanori Kobayashi, and Takao Ojima. 2014. "Characterization of an Alginate Lyase, FlAlyA, from Flavobacterium sp. Strain UMI-01 and Its Expression in Escherichia coli" Marine Drugs 12, no. 8: 4693-4712. https://doi.org/10.3390/md12084693
APA StyleInoue, A., Takadono, K., Nishiyama, R., Tajima, K., Kobayashi, T., & Ojima, T. (2014). Characterization of an Alginate Lyase, FlAlyA, from Flavobacterium sp. Strain UMI-01 and Its Expression in Escherichia coli. Marine Drugs, 12(8), 4693-4712. https://doi.org/10.3390/md12084693




