Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Characterizations
2.1.1. Molecular Weight Measurement

| Fucoidans | Mn (kDa) | Mw (kDa) | Mn/Mw |
|---|---|---|---|
| Crude | 3.5 ± 0.4 | 6.2 ± 0.3 | 1.5 ± 0.2 |
| Purified | 4.9 ± 0.2 | 7.5 ± 0.2 | 1.5 ± 0.1 |
2.1.2. Chemical Composition
| Fucoidans | l-Fucose | Uronic Acid | Sulfate | Other |
|---|---|---|---|---|
| Crude | 25.0% ± 0.5% | 16.1% ± 0.9% | 21.7% ± 2.0% | 37.2% |
| Purified | 42.8% ± 2.2% | 16.9% ± 0.4% | 25.1% ± 0.8% | 15.2% |
2.1.3. FTIR Analysis

2.1.4. NMR Analysis

2.2. In Vivo Studies of the 99mTc-Radiolabeled Fucoidans
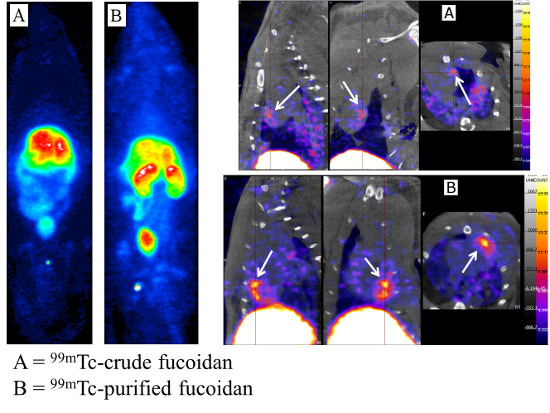
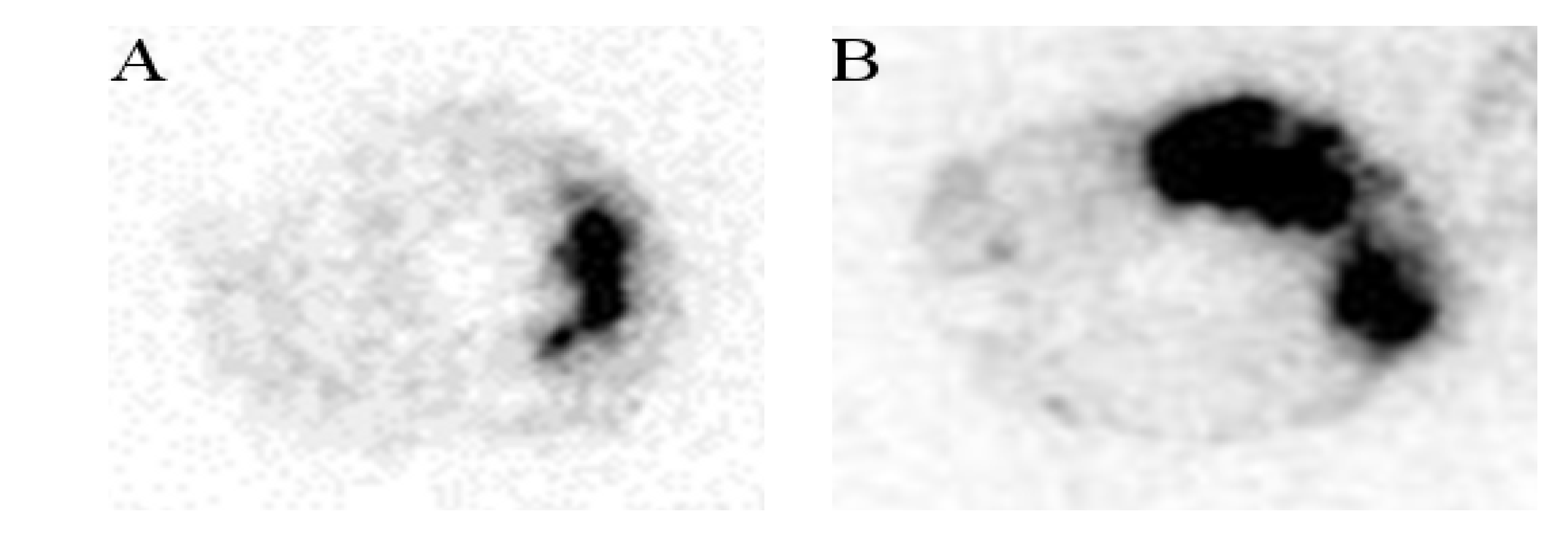
2.2.1. Biodistribution Studies of the Radiolabeled Fucoidans


2.2.2. Myocardial Ischemia-Reperfusion

3. Discussions
4. Experimental Section
4.1. Polysaccharide and Common Chemicals
4.2. Molecular Weight Determination
4.3. Purification of Fucoidan
4.4. Colorimetric Assay
4.4.1. Fucose
4.4.2. Glucuronic Acid
4.4.3. Sulfate
4.5. Infrared Spectra
4.6. Nuclear Magnetic Resonance (NMR)
4.7. Radiolabeling Procedure
4.8. Biodistribution and Experimental Model of Myocardial Ischemia-Reperfusion
4.9. Imaging Procedures
4.9.1. Acquisition and Reconstruction Parameters
4.9.2. Data Analysis
4.10. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945. [Google Scholar] [CrossRef]
- Lalatonne, Y.; Paris, C.; Serfaty, J.M.; Weinmann, P.; Lecouvey, M.; Motte, L. Bis-phosphonates—Ultra small superparamagnetic iron oxide nanoparticles: A platform towards diagnosis and therapy. Chem. Commun. 2008, 22, 2553–2555. [Google Scholar] [CrossRef]
- Corr, S.A.; O’Byrne, A.; Gun’ko, Y.K.; Ghosh, S.; Brougham, D.F.; Mitchell, S.; Volkov, Y.; Prina-Mello, A. Magnetic-fluorescent nanocomposites for biomedical multitasking. Chem. Commun. 2006, 43, 4474–4476. [Google Scholar] [CrossRef]
- Masotti, A.; Pitta, A.; Ortaggi, G.; Corti, M.; Innocenti, C.; Lascialfari, A.; Marinone, M.; Marzola, P.; Daducci, A.; Sbarbati, A.; et al. Synthesis and characterization of polyethylenimine-based iron oxide composites as novel contrast agents for MRI. Magn. Resonance Mater. Phys. Biol. Med. 2009, 22, 77–87. [Google Scholar]
- Petri-Fink, A.; Chastellain, M.; Juillerat-Jeanneret, L.; Ferrari, A.; Hofmann, H. Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials 2005, 26, 2685–2694. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Niu, G.; Huang, J.; Chen, K.; Li, X.; Chen, X. Human serum albumin coated iron oxide nanoparticles for efficient cell labeling. Chem. Commun. 2010, 46, 433–435. [Google Scholar] [CrossRef]
- Tsourkas, A.; Shinde-Patil, V.R.; Kelly, K.A.; Patel, P.; Wolley, A.; Allport, J.R.; Weissleder, R. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug. Chem. 2005, 16, 576–581. [Google Scholar] [CrossRef]
- Gao, F.; Cai, Y.; Zhou, J.; Xie, X.; Ouyang, W.; Zhang, Y.; Wang, X.; Zhang, X.; Wang, X.; Zhao, L.; Tang, J. Pullulan acetate coated magnetite nanoparticles for hyper-thermia: Preparation, characterization and in vitro experiments. Nano Res. 2010, 3, 23–31. [Google Scholar] [CrossRef]
- Molday, R.S.; MacKenzie, D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J. Immunol. Methods 1982, 52, 353–367. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI-PEG-Chitosan-Copolymer-Coated iron oxide nanoparticles for safe gene delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef]
- Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1971–1979. [Google Scholar]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014. [Google Scholar] [CrossRef]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef]
- Blondin, C.; Fischer, E.; Boisson-Vidal, C.; Kazatchkine, M.D.; Jozefonvicz, J. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol. Immunol. 1994, 31, 247–253. [Google Scholar] [CrossRef]
- Tissot, B.; Daniel, R. Biological properties of sulfated fucans: The potent inhibiting activity of algal fucoidan against the human complement system. Glycobiology 2003, 13, 29G–30G. [Google Scholar] [CrossRef]
- Bachelet, L.; Bertholon, I.; Lavigne, D.; Vassy, R.; Jandrot-Perrus, M.; Chaubet, F.; Letourneur, D. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 141–146. [Google Scholar]
- Rouzet, F.; Bachelet-Violette, L.; Alsac, J.M.; Suzuki, M.; Meulemans, A.; Louedec, L.; Petiet, A.; Jandrot-Perrus, M.; Chaubet, F.; Michel, J.B.; et al. Radiolabeled fucoidan as a P-Selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J. Nucl. Med. 2011, 52, 1433–1440. [Google Scholar]
- Suzuki, M.; Bachelet-Violette, L.; Rouzet, F.; Beilvert, A.; Autret, G.; Maire, M.; Menager, C.; Louedec, L.; Choqueux, C.; Saboural, P.; et al. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine 2014. [Google Scholar] [CrossRef]
- Bachelet-Violette, L.; Silva, A.K.A.; Maire, M.; Michel, A.; Brinza, O.; Ou, P.; Ollivier, V.; Nicoletti, A.; Wilhelm, C.; Letourneur, D.; et al. Strong and specific interaction of ultra small superparamagnetic iron oxide nanoparticles and human activated platelets mediated by fucoidan coating. RSC Adv. 2014, 4, 4864–4871. [Google Scholar]
- Bernardi, G.; Springer, G.F. Properties of highly purified fucan. J. Biol. Chem. 1962, 237, 75–80. [Google Scholar]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar]
- Choi, J.; Gu Lee, S.; Jong Han, S.; Cho, M.; Cheon Lee, P. Effect of gamma irradiation on the structure of fucoidan. Radiat. Phys. Chem. 2014, 100, 54–58. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Kylin, H. Zur Biochemie der Meeresalgen. Z. Für Physiol. Chem. 1913, 83, 171–197. [Google Scholar]
- Kloareg, B.; Quatrano, R. S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. In Oceanography and Marine Biology; Barnes, M., Ed.; Aberdeen University Press: Aberdeen, UK, 1988; Volume 26, pp. 259–315. [Google Scholar]
- Chaubet, F.; Chevolot, L.; Jozefonvicz, J.; Durand, P.; Boisson-Vidal, C. Relationships between chemical characteristics and anticoagulant activity of low molecular weight fucans from marine algae. In Bioactive Carbohydrate Polymers; Paulsen, B.S., Ed.; Springer: Heidelberg, Germany, 2000; Volume 44, pp. 59–84. [Google Scholar]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifantiev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar]
- Leite, E.L.; Medeiros, M.G.L.; Rocha, H.A.O.; Farias, G.G.M.; da Silva, L.F.; Chavante, S.F.; de Abreu, L.D.; Dietrich, C.P.; Nader, H.B. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schroederi. Plant Sci. 1998, 132, 215–228. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Chevolot, L.; Varenne, A.; Gareil, P.; Goasdoue, N. Regioselective desulfation of sulfated l-fucopyranoside by a new sulfoesterase from the marine mollusk Pecten maximus—Application to the structural study of algal fucoidan (Ascophyllum nodosum). Eur. J. Biochem. 2001, 268, 5617–5626. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Kulkarni, P.V.; Parkey, R.W.; Wilson, J.E.; Lewis, S.E.; Buja, L.M.; Bonte, F.J.; Willerson, J.T. Modified Technetium-99m Heparin for the Imaging of Acute Experimental Myocardial Infarcts. J. Nucl. Med. 1980, 21, 117–121. [Google Scholar]
- Dische, Z. New Color Reactions for Determination of Sugars in Polysaccharides. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1955; Volume 2, pp. 313–358. [Google Scholar]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar]
- Gustafsson, L. Determination of ultramicro amounts of sulphate as methylene blue—II The reduction. Talanta 1960, 4, 236–243. [Google Scholar] [CrossRef]
- Kuban, V.; Dasgupta, P.K.; Marx, J.N. Nitroprusside and methylene blue methods for silicone membrane differentiated flow injection determination of sulfide in water and wastewater. Anal. Chem. 1992, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Spies, H.; Pietzsch, H.J. Stannous Chloride in the Preparation of 99mTc Pharmaceuticals. In Technetium-99m Pharmaceuticals; Zolle, I., Ed.; Springer Berlin: Heidelberg, Germany, 2007; pp. 59–66. [Google Scholar]
- Saffari, H.; Krstyen, J.J.; Gonzalez, C.; Clayton, F.C.; Leiferman, K.M.; Gleich, G.J.; Peterson, K.A.; Pease, L.F. 99mTechnetium-labeled heparin: A new approach to detection of eosinophilic esophagitis-associated inflammation. J. Allergy Clin. Immunol. 2013, 132, 1446–1448. [Google Scholar] [CrossRef]
- Dass, R.S.; Singh, A.K.; Chauhan, U.P. Development of a dextran kit for labelling with 99mTc and its evaluation for lymphoscintigraphy. Nucl. Med. Biol. 1993, 20, 701–706. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saboural, P.; Chaubet, F.; Rouzet, F.; Al-Shoukr, F.; Azzouna, R.B.; Bouchemal, N.; Picton, L.; Louedec, L.; Maire, M.; Rolland, L.; et al. Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction. Mar. Drugs 2014, 12, 4851-4867. https://doi.org/10.3390/md12094851
Saboural P, Chaubet F, Rouzet F, Al-Shoukr F, Azzouna RB, Bouchemal N, Picton L, Louedec L, Maire M, Rolland L, et al. Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction. Marine Drugs. 2014; 12(9):4851-4867. https://doi.org/10.3390/md12094851
Chicago/Turabian StyleSaboural, Pierre, Frédéric Chaubet, Francois Rouzet, Faisal Al-Shoukr, Rana Ben Azzouna, Nadia Bouchemal, Luc Picton, Liliane Louedec, Murielle Maire, Lydia Rolland, and et al. 2014. "Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction" Marine Drugs 12, no. 9: 4851-4867. https://doi.org/10.3390/md12094851
APA StyleSaboural, P., Chaubet, F., Rouzet, F., Al-Shoukr, F., Azzouna, R. B., Bouchemal, N., Picton, L., Louedec, L., Maire, M., Rolland, L., Potier, G., Guludec, D. L., Letourneur, D., & Chauvierre, C. (2014). Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction. Marine Drugs, 12(9), 4851-4867. https://doi.org/10.3390/md12094851