Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases
Abstract
:1. Introduction
2. Neurodegenerative Diseases: Alzheimer’s and Parkinson’s
2.1. Mechanisms of Neurodegeneration
2.1.1. Neuroinflammation
2.1.2. Oxidative/Nitrosative Damage
2.1.3. Synaptic Loss
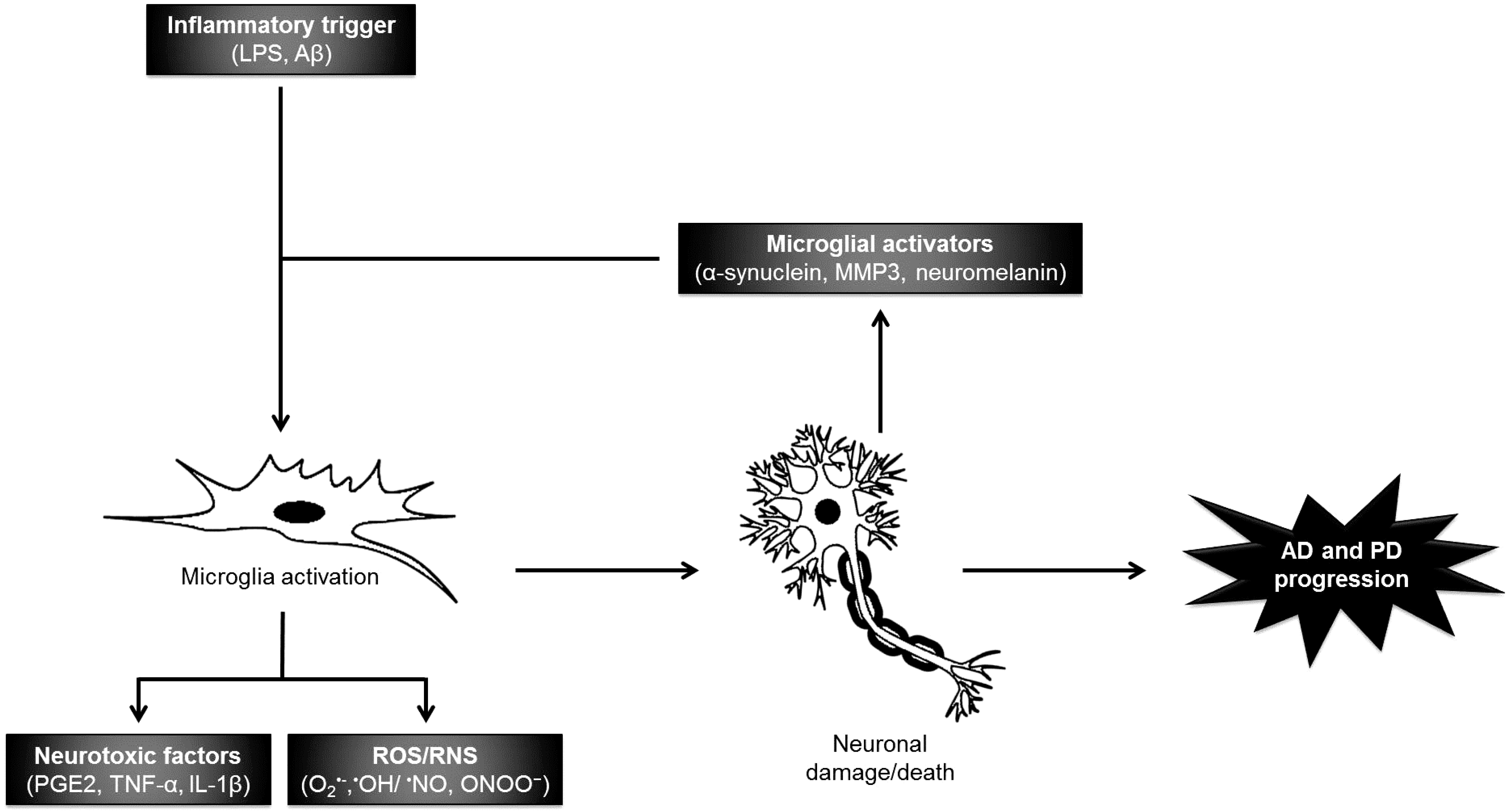
3. Neuroprotective Compounds from Macroalgae
3.1. Phlorotannins





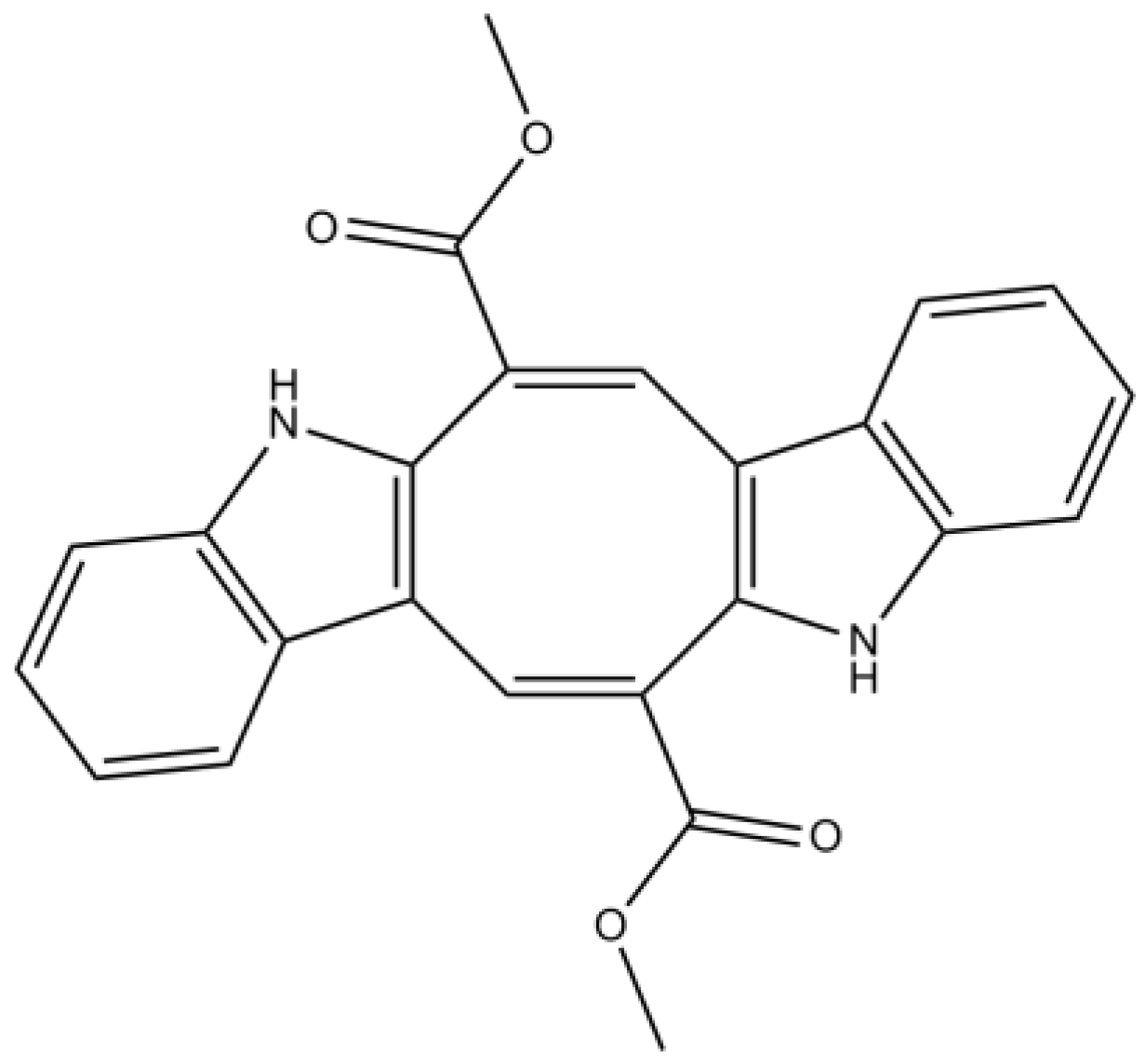
3.2. Alkaloids

3.3. Terpenes






3.4. Pigments



3.5. Sterols

3.6. Oligo- and Polysaccharides


3.7. Fatty Acids

3.8. Other Compounds

4. Conclusions
| Class | Compound | Neuroprotective Effects | References | |
|---|---|---|---|---|
| Simple phenol | Phloroglucinol | Suppression of the overproduction of intracellular ROS, decrease of intracellular Ca2+ levels and reduction of apoptosis | [35] | |
| Phlorotannins | Dieckol | Inhibitory activity against AChE | [67] | |
| Reduction of the expression and release of •NO, PGE2, IL-1β and TNF-α in microglial cells | [29] | |||
| Suppression of the overproduction of intracellular ROS, decrease of intracellular Ca2+ levels and reduction of apoptosis | [35] | |||
| Phlorofucofuroeckol | Inhibitory activity against AChE | [59] | ||
| Phlorofucofuroeckol A | Inhibitory activity against AChE and BuChE | [60] | ||
| Suppression of intracellular ROS generation and decrease of Ca2+ levels | [62] | |||
| Eckol | Inhibitory activity against AChE | [60,67] | ||
| Suppression of the overproduction of intracellular ROS, decrease of intracellular Ca2+ levels and reduction of apoptosis | [35] | |||
| 2-Phloroeckol | Inhibitory activity against AChE | [60] | ||
| 7-Phloroeckol | Inhibitory activity against AChE | [60] | ||
| Suppression of intracellular ROS generation and decrease of Ca2+ levels | [62] | |||
| Phlorotannins | Eckstolonol | Inhibitory activity against AChE and BuChE | [60] | |
| Suppression of the overproduction of intracellular ROS, decrease of intracellular Ca2+ levels and reduction of apoptosis | [35] | |||
| 6,6′-Bieckol | Potent inhibitory activity against AChE (non-competitive inhibition type) | [61] | ||
| DPHC | Moderate inhibitory activity against BuChE | [61] | ||
| Antioxidant mechanisms and control of intracellular Ca2+ levels | [38] | |||
| Triphlorethol A | Suppression of the overproduction of intracellular ROS, decrease of intracellular Ca2+ levels and reduction of apoptosis | [35] | ||
| Fucophlorethol A | Scavenging of reactive carbonyls, inhibiting the formation of AGEs | [66] | ||
| Tetrafucol A | Scavenging of reactive carbonyls, inhibiting the formation of AGEs | [66] | ||
| Trifucodiphlorethol A | Scavenging of reactive carbonyls, inhibiting the formation of AGEs | [66] | ||
| Dibenzo [1,4] dioxine-2,4,7,9-tetraol | Inhibitory activity against AChE | [67] | ||
| Alkaloids | Caulerpin | Antioxidant properties | [74] | |
| Moderate/weak attenuation of Aβ-induced SH-SY5Y cell damage (unknown mechanism) | [73] | |||
| Racemosin A | Strong attenuation of Aβ-induced SH-SY5Y cell damage (unknown mechanism) | [73] | ||
| Racemosin B | Moderate/weak attenuation of the Aβ-induced SH-SY5Y cell damage (unknown mechanism) | [73] | ||
| Terpenes | Meroterpenes | Sargachromenol | Promotion of NGF–dependent neurogenesis by stabilization of the microtubule assembling and extension of neuritis via PKA and MAPK signaling pathways | [81] |
| Moderate inhibitory activity against AChE | [87] | |||
| Sargaquinoic acid | Enhancement of neuritis outgrowth via TrkA‑MAPK and adenylate cyclase‑PKA signaling pathways | [84] | ||
| Antioxidant properties | [85] | |||
| Potent inhibitory activity against BuChE and moderate inhibitory activity against AChE | [87] | |||
| Meroditerpenes | Epitaondiol | Inhibition of PLA2 and COX pathway | [88] | |
| Stypotriol triacetate | ||||
| Sesquiterpenes | Pacifenol | Inhibition of PLA2 and COX pathway | [88] | |
| (5E,10Z)-6,10,14-trimethylpentadeca-5,10-dien-2,12-dione | Moderate inhibitory activity against AChE and BuChE | [89] | ||
| (5E,9E,13E)-6,10,14-trimethylpentadeca-5,9,13-trien-2,12-dione | ||||
| Caulerpenyne | Inhibitory activity against LOX | [90] | ||
| Carotenoids | Fucoxanthin | Attenuation of neuronal cell damage through scavenging activity | [98] | |
| Inhibition of intracellular ROS formation, DNA damage, and apoptosis induced by H2O2 | [101] | |||
| Suppression of inflammation and oxidative damage in microglial cells via inhibition of MAPK pathway | [102] | |||
| Carotenoids | AST | Suppression of expression and formation of •NO, iNOS and COX-2 | [104] | |
| Suppression of intracellular ROS generation, mitochondrial dysfunctions and p38 MAPK pathway | [105] | |||
| Antioxidant properties | [107,108] | |||
| Reduction of the expression of IL-6 via inhibition of MAPK signaling pathway | [109] | |||
| Suppression of MPP+/MPTP-induced mitochondrial dysfunction and ROS production via up-regulation of the expression of Bcl-2 protein, down-regulation of the expression of Bax and α-synuclein, and inhibition of the activation of caspase-3 | [110] | |||
| Suppression of ROS production and inhibition of Sp1/NR1 signaling pathway | [111] | |||
| Chlorophyll derivatives | Pheophytin A | Enhancement of neuritis outgrowth via the activation of MAPK pathway | [113] | |
| Potent inhibitory activity against LOX enzymes | [115] | |||
| Sterols | Fucosterol | Moderate inhibitory activity against BuChE | [60] | |
| Inhibitory activity against AChE and BuChE | [9] | |||
| Phycobiliproteins | C-PC | Scavenge of numerous radicals and inhibition of lipid peroxidation, preventing oxidative damage | [117,118,119,120,121,122] | |
| Protection against iron-induced SH-SY5Y toxicity through the increase of cellular antioxidant enzymes (GPx, GR, GPx-Se) and GSH levels | [123] | |||
| Protection of hippocampus neurons induced by global cerebral ischemia/reperfusion injury through the reduction of ROS levels and possible inhibition of acute microglia activation | [124] | |||
| Oligo- and Polysaccharides | Fucoidan | Suppression of ROS generation and activation of caspase-9 and caspase-3 | [130] | |
| Antioxidant properties | [133] | |||
| Activation of PI3K/Akt survival pathway | [136] | |||
| κ-carrageenan | Inhibition of the viability and content of •NO, TNF-α and IL-10 released by LPS-activated microglia cells | [139,141] | ||
| Fatty acids | EPA | Moderate inhibitory activity against AChE | [9,159] | |
| Anti-inflammatory activity of EPA-derived products (resolvin E1, and 5- and 18-hydroxy-EPA) | [165] | |||
| DHA | Inhibitory activity against AChE | [9,159] | ||
| Promotion of neuronal survival by positive modulation of Akt | [160] | |||
| Enhancement of neuritis growth and synaptogenesis by DHA ethanolamide metabolites | [162] | |||
| DHA-derived mediator (NPD 1) promotes cell survival presumably through the up-regulation of Bcl-2 and Bcl-xL, down-regulation of Bax and Bad, suppression of oxidative stress-induced caspase-3 activation and IL-1-stimulated expression of COX-2 | [163] | |||
| Glycerol glycosides | Floridoside | Suppression of pro-inflammatory responses in microglia through the inhibition of the production of •NO and ROS and blockage of MAPK pathway | [32] | |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I.1. J. Org. Chem. 1951, 16, 981–987. [Google Scholar]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades – where and what are we bioprospecting? PLoS One 2012, 7, e30580. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid–liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 7, 1765–1772. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- McHugh, D.J. A guide to the seaweed industry. In FAO Fisheries Technical Paper No. 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 1–105. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Wijesinghe, W.A.J.P.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type ІІ diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Kamada, T.; Lee, W.-W.; Jeon, Y.-J. Anti-inflammatory activity of halogenated secondary metabolites of Laurencia snackeyi (Weber-van Bosse) Masuda in LPS-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1805–1813. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.; Nishigaki, I. Cancer preventive efficacy of marine carotenoid fucoxanthin: Cell cycle arrest and apoptosis. Nutrients 2013, 5, 4978–4989. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.A.; Siraj, A.; Inamdar, N.N. Pharmacotherapeutic approaches of Parkinson’s disease. Int. J. Pharmacol. 2010, 6, 584–590. [Google Scholar]
- Cho, S.; Kim, S.-K. Neuropharmacological properties of marine plants. In Marine Pharmacognosy: Trends and Applications; Kim, S.-K., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 355–372. [Google Scholar]
- Holden, M.; Kelly, C. Use of cholinesterase inhibitors in dementia. Adv. Psychiatr. Treat. 2002, 8, 89–96. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 2, 208–245. [Google Scholar]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Assessment of anticholinesterase activity of Gelidiella acerosa: Implications for its therapeutic potential against Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar]
- Kang, I.-J.; Jang, B.G.; In, S.; Choi, B.; Kim, M.; Kim, M.-J. Phlorotannin-rich Ecklonia cava reduces the production of beta-amyloid by modulating alpha- and gamma-secretase expression and activity. Neuro Toxicol. 2013, 34, 16–24. [Google Scholar]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Biundo, R.; Weis, L.; Facchini, S.; Formento-Dojot, P.; Vallelunga, A.; Pilleri, M.; Antonini, A. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Park. Relat. Disord. 2014, 20, 394–399. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. New therapeutic strategy for Parkinson’s and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.; Block, M. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Smid, S.D.; Maag, J.L.; Musgrave, I.F. Dietary polyphenol-derived protection against neurotoxic β-amyloid protein: From molecular to clinical. Food Funct. 2012, 3, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Ahn, Y.-W.; Lee, S.-H.; Choi, Y.H.; Kim, S.-K.; Yea, S.S.; Choi, I.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-κB pathways. Food Chem. Toxicol. 2009, 47, 410–417. [Google Scholar]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef]
- Jung, W.-K.; Heo, S.-J.; Jeon, Y.-J.; Lee, C.-M.; Park, Y.-M.; Byun, H.-G.; Choi, Y.H.; Park, S.-G.; Choi, I.-W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- In’t Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.B.; Stricker, B.H.C. Nonsteroidal anti-inflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. Biochem. Mol. Biol. Rep. 2013, 46, 398–403. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. The use of flavonoids in central nervous system disorders. Curr. Med. Chem. 2013, 20, 4694–4719. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Cha, S.-H.; Ko, J.-Y.; Kang, M.-C.; Kim, D.; Heo, S.-J.; Kim, J.-S.; Heu, M.S.; Kim, Y.-T.; Jung, W.-K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Kim, K.-N.; Lee, S.-H.; Ahn, G.; Kang, D.-H.; Oh, C.; Choi, Y.-U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.H. Neuroprotective molecular mechanisms of (−)-epigallocatechin-3-gallate: A reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef]
- Bazelyansky, M.; Robey, E.; Kirsch, J.F. Fractional diffusion-limited component of reactions catalyzed by acetylcholinesterase. Biochemistry 1986, 25, 125–130. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Suganthy, N.; Karutha Pandian, S.; Pandima Devi, K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.-J.; Jeon, Y.E.; Yin, X.F.; Nam, J.-S.; You, S.G.; Hong, M.S.; Jang, B.G.; Kim, M.-J. Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid, and reduces beta-amyloid mediated neuronal death. Food Chem. Toxicol. 2011, 49, 2252–2259. [Google Scholar]
- Xu, P.-X.; Wang, S.-W.; Yu, X.-L.; Su, Y.-J.; Wang, T.; Zhou, W.-W.; Zhang, H.; Wang, Y.-J.; Liu, R.-T. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G. Seaweeds from the Portuguese Coast: Chemistry, Antimicrobial and Anti-inflammatory Capacity. Ph.D. Thesis, Phytochemistry and Pharmacognosy Speciality, Faculty of Pharmacy, University of Porto, Porto, Portugal, January 2014. [Google Scholar]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS One 2012, 7, e31145. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Yamada, Y.; Imai, K.; Ito, T.; Kakinuma, M.; Amano, H. The inhibitory effects of components from a brown alga, Eisenia arborea, on degranulation of mast cells and eicosanoid synthesis. J. Funct. Foods 2009, 1, 387–393. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Jung, W.-S.; Song, D.-G.; Um, B.-H.; Son, J.-K.; Pan, C.-H. Phlorofucofuroeckol-A, a potent inhibitor of aldo-keto reductase family 1 member B10, from the edible brown alga Eisenia bicyclis. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 721–727. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.-S.; Shin, H.-C.; Bao, H.; Yeo, S.; Lee, B.; Kang, J. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.S. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Lee, S.-H.; Yong, L.; Kim, S.-K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Robino, G.; Obbili, A.; Bardini, P.; Aragno, M.; Parola, M.; Danni, O. H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp. Neurol. 2003, 180, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Kim, S.-K. Utilization of seaweed derived ingredients as potential antioxidants and functional ingredients in the food industry: An overview. Food Sci. Biotechnol. 2011, 20, 1461–1466. [Google Scholar] [CrossRef]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; van Staden, J. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Naili, M. In vitro antibacterial activity of alkaloid extracts from green, red and brown macroalgae from western coast of Libya. Afr. J. Biotechnol. 2013, 5, 7086–7091. [Google Scholar]
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Güven, K.; Coban, B.; Sezik, E.; Erdugan, H.; Kaleağasıoğlu, F. Alkaloids of marine macroalgae. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer (Berlin Heidelberg): Berlin, Germany, 2013; pp. 25–37. [Google Scholar]
- Kaleağasıoğlu, F.; Güven, K.; Sezik, E.; Erdugan, H.; Coban, B. Pharmacology of macroalgae alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2013; pp. 1203–1216. [Google Scholar]
- Liu, D.-Q.; Mao, S.-C.; Yu, X.-Q.; Feng, L.-H.; Lai, X.-P. Caulerchlorin, a novel chlorinated bisindole alkaloid with antifungal activity from the Chinese green alga Caulerpa racemosa. Heterocycles 2012, 85, 661–666. [Google Scholar] [CrossRef]
- Liu, D.-Q.; Mao, S.-C.; Zhang, H.-Y.; Yu, X.-Q.; Feng, M.-T.; Wang, B.; Feng, L.-H.; Guo, Y.-W. Racemosins A and B, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. [Google Scholar] [CrossRef] [PubMed]
- De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.; Prates Lorenzo, V.; de Miranda, G.E.; de Araújo-Júnior, J.X.; de Oliveira Chaves, M.C.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar]
- Miglio, G.; Varsaldi, F.; Francioli, E.; Battaglia, A.; Canonico, P.L.; Lombardi, G. Cabergoline protects SH-SY5Y neuronal cells in an in vitro model of ischemia. Eur. J. Pharmacol. 2004, 489, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Vinholes, J.; Valentão, P.; Andrade, P.B. Halogenated compounds from seaweed, a biological overview. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Incorporated: New York, NY, USA, 2011; pp. 163–184. [Google Scholar]
- De las Heras, B.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy – Drug Targets 2009, 8, 28–39. [Google Scholar]
- Gross, H.; König, G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006, 11, 115–141. [Google Scholar] [CrossRef]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, biological role, and therapeutic applications. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer (Berlin Heidelberg): Berlin, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Wang, G.; Tang, W.; Bidigare, R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Zhang, L., Demain, A., Eds.; Humana Press: New York, NY, USA, 2005; pp. 197–227. [Google Scholar]
- Tsang, C.K.; Ina, A.; Goto, T.; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Connor, B.; Dragunow, M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Rev. 1998, 27, 1–39. [Google Scholar] [PubMed]
- Heese, K.; Low, J.W.; Inoue, N. Nerve growth factor, neural stem cells and Alzheimer’s disease. Neuro-Signals 2006, 15, 1–12. [Google Scholar]
- Kamei, Y.; Tsang, C.K. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int. J. Dev. Neurosci. 2003, 21, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Kamei, Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur. J. Pharmacol. 2004, 488, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; McGeer, E.G.; McGeer, P.L. Therapeutic approaches to inflammation in neurodegenerative disease. Curr. Opin. Neurol. 2007, 20, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Ryu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; de Girolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Park, S.H.; Kim, E.S.; Choi, B.W.; Ryu, S.Y.; Lee, B.H. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch. Pharm. Res. 2003, 26, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, S.; Cavas, L.; Yurdakoc, K.; Pohnert, G. The sesquiterpene caulerpenyne from Caulerpa spp. is a lipoxygenase inhibitor. Mar. Biotechnol. 2011, 13, 321–326. [Google Scholar] [CrossRef]
- Manev, H.; Chen, H.; Dzitoyeva, S.; Manev, R. Cyclooxygenases and 5-lipoxygenase in Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 315–319. [Google Scholar]
- Chu, J.; Praticò, D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer’s disease transgenic mouse model: Involvement of γ-secretase. Am. J. Pathol. 2011, 178, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Abrahamson, E.E.; Uz, T.; Manev, H.; DeKosky, S.T. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J. Histochem. Cytochem. 2008, 56, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Kim, H.J.; Chun, H.S. Quantitative structure−activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 2007, 80, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingedients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Zorofchian Moghadamtousi, S.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Abdul Kadir, H. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Ikeda, K.; Kitamura, A.; Machida, H.; Watanabe, M.; Negishi, H.; Hiraoka, J.; Nakano, T. Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Kang, H.-S.; Kim, J.-P.; Kim, S.-H.; Lee, K.-W.; Cho, M.-G.; Jeon, Y.-J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Pangestuti, R.; Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Fucoxanthin ameliorates inflammation and oxidative responses in microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Park, Y.S.; Choi, D.K.; Chang, H.I. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [PubMed]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Bioactive marine drugs and marine biomaterials for brain diseases. Mar. Drugs 2014, 12, 2539–2589. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Lee, Y.J.; Kwon, K.H. Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J. Clin. Biochem. Nutr. 2010, 47, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Koh, H.K.; Kim, D.S. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, C.S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhang, X.; Huang, B.; Zhu, Y.; Chen, X. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar. Drugs 2013, 11, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.-I.; Nozaki, H.; Kamei, Y. Pheophytin A, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Khodosevich, K.; Monyer, H. Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons in vitro. BMC Neurosci. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Kagawa, Y.; Konno, R.; Kim, S.M.; Takahashi, K. Lipoxygenase inhibitors derived from marine macroalgae. Bioorganic Med. Chem. Lett. 2014, 24, 1383–1385. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Hayes, M., Ed.; Springer US: New York, NY, USA, 2012; pp. 55–98. [Google Scholar]
- Romay, C.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.B.; Madyastha, K.M. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pinero Estrada, J.E.; Bermejo Bescos, P.; Villar del Fresno, A.M. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco 2001, 56, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.C.; Jouy, N.; Brune, J.P.; Oreal, H.; Cristol, J.P.; Rouanet, J.M. Phycobiliprotein C-phycocyanin from Spirulina platensisis powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Pinero, E.; Villar, A.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008, 110, 436–445. [Google Scholar] [CrossRef]
- Upasani, C.D.; Balaraman, R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous. Phytother. Res. 2003, 17, 330–334. [Google Scholar]
- Bermejo-Bescós, P.; Piñero-Estrada, E.; Villar del Fresno, Á.M. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro 2008, 22, 1496–1502. [Google Scholar]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Bernardo, J.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Mouga, T. Sterol profiles in 18 macroalgae of the Portuguese coast. J. Phycol. 2011, 47, 1210–1218. [Google Scholar] [CrossRef]
- Schwender, J.; Gemünden, C.; Lichtenthaler, H.K. Chlorophyta exclusively use the 1-deoxyxylulose 5-phosphate⁄2-C-methylerythritol 4-phosphate pathway for the biosynthesis of isoprenoids. Planta 2001, 212, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Schwender, J.; Polle, J.E.W. Isoprenoid biosynthesis in eukaryotic phototrophs: A spotlight on algae. Plant Sci. 2012, 185–186, 9–22. [Google Scholar]
- Lopes, G.; Sousa, C.; Valentão, P.; Andrade, P.B. Sterols in algae and health. In Bioactive Compounds from Marine Foods; Hernández-Ledesma, B., Herrero, M., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2013; pp. 173–191. [Google Scholar]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.; Imbs, T.; Shevchenko, N.; Spirin, P.; Horn, S.; Fehse, B.; Zvyagintseva, T.; Prassolov, V. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin. Exp. Pharm. Physiol. 2009, 37, 422–428. [Google Scholar] [CrossRef]
- Cui, Y.-Q.; Jia, Y.-J.; Zhang, T.; Zhang, Q.-B.; Wang, X.-M. Fucoidan protects against lipopolysaccharide-induced rat neuronal damage and inhibits the production of pro-inflammatory mediators in primary microglia. CNS Neurosci. Ther. 2012, 18, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar]
- Yu, G.; Guan, H.; Ioanoviciu, A.S.; Sikkander, S.A.; Thanawiroon, C.; Tobacman, J.K.; Toida, T.; Linhardt, R.J. Structural studies on κ-carrageenan derived oligosaccharides. Carbohydr. Res. 2002, 337, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tao, H.; Xie, J.; Zhang, S.; Xu, X. Degradation and antioxidant activity of κ-carrageenans. J. Appl. Polym. Sci. 2010, 117, 194–199. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Z.; Wu, H.; Wang, F.; Zhang, S. The immune regulation of κ-carrageenan oligosaccharide and its desulfated derivatives on LPS-activated microglial cells. Neurochem. Int. 2012, 61, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-A.; Xu, L.; Wu, H.-G. Immunomodulatory function of κ-carrageenan oligosaccharides acting on LPS-activated microglial cells. Neurochem. Res. 2014, 39, 333–343. [Google Scholar]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nobre, M.E.P.; Correia, A.O.; Borges, M.D.B.; Sampaio, T.M.A.; Chakraborty, S.A.; Gonçalves, D.D.O.; Brito, G.A.D.C.; Leal, L.K.A.M.; Felipe, C.F.B.; Lucetti, D.L.; et al. Eicosapentaenoic acid and docosahexaenoic acid exert anti-inflammatory and antinociceptive effects in rodents at low doses. Nutr. Res. 2013, 33, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar]
- Colombo, M.L.; RisÈ, P.; Giavarini, F.; Angelis, L.; Galli, C.; Bolis, C.L. Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods Hum. Nutr. 2006, 61, 64–69. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Essential fatty acids and the brain: Possible health implications. Int. J. Dev. Neurosci. 2000, 18, 383–399. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Bornebroek, M.; Witteman, J.C.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. J. Mol. Neurosci. 2001, 16, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Murthy, M.; Hamilton, J.; Greiner, R.S.; Moriguchi, T.; Salem, N., Jr.; Kim, H.-Y. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J. Lipid Res. 2002, 43, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.M.; Bergqvist, F.; Ivanova, E.V.; Jacobsen, K.T.; Iverfeldt, K. Exposure to the saturated free fatty acid palmitate alters BV-2 microglia inflammatory response. J. Mol. Neurosci. 2013, 51, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Lipid Replacement as an adjunct to therapy for chronic fatigue, anti-aging and restoration of mitochondrial function. J. Am. Nutraceutical Assoc. 2003, 6, 4–10. [Google Scholar]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Biophys. Acta 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ellithorpe, R. Lipid replacement and antioxidant nutritional therapy for restoring mitochondrial function and reducing fatigue in chronic fatigue syndrome and other fatiguing illnesses. J. Chronic Fatigue Syndr. 2006, 13, 57–68. [Google Scholar] [CrossRef]
- Silva, G.; Pereira, R.B.; Valentão, P.; Andrade, P.B.; Sousa, C. Distinct fatty acid profile of ten brown macroalgae. Rev. Bras. Farmacogn. 2013, 23, 608–613. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D. Fatty acid contents and profiles of 16 macroalgae collected from the Irish Coast at two seasons. J. Appl. Phycol. 2014, 26, 451–463. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Ren, Y.; Houghton, P.; Hider, R.C. Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer’s disease. J. Pharm. Pharmacol. 2006, 58, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.-Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Ertley, R.N.; Lee, H.J.; DeMar, J.C.J.; Arnold, J.T.; Rapoport, S.I.; Bazinet, R.P. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry 2007, 12, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, D.M.; Akbar, M.; Huang, B.X. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Palumbo, S.; Bosetti, F.; Mount, H.T.; Kang, J.X.; Greenwood, C.E.; Ma, D.W.L.; Serhan, C.N.; Bazinet, R.P. Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 2013, 127, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Liu, Z.; Bazinet, R.P. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: An intracerebroventricular study. J. Neurochem. 2011, 116, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.K.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. In Nineteenth International Seaweed Symposium; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtum, K., Notoya, M., Eds.; Springer (Netherlands): Dordrecht, The Netherlands, 2009; Volume 2, pp. 159–167. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs 2014, 12, 4934-4972. https://doi.org/10.3390/md12094934
Barbosa M, Valentão P, Andrade PB. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Marine Drugs. 2014; 12(9):4934-4972. https://doi.org/10.3390/md12094934
Chicago/Turabian StyleBarbosa, Mariana, Patrícia Valentão, and Paula B. Andrade. 2014. "Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases" Marine Drugs 12, no. 9: 4934-4972. https://doi.org/10.3390/md12094934
APA StyleBarbosa, M., Valentão, P., & Andrade, P. B. (2014). Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Marine Drugs, 12(9), 4934-4972. https://doi.org/10.3390/md12094934






