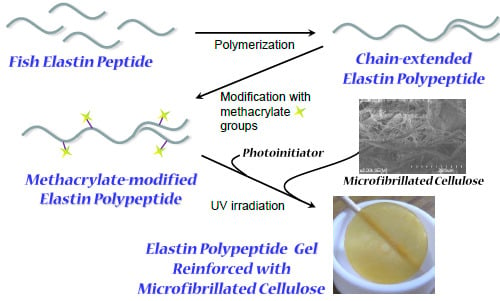
Preparation of Photocrosslinked Fish Elastin Polypeptide/Microfibrillated Cellulose Composite Gels with Elastic Properties for Biomaterial Applications
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of a Methacrylate-Modified Fish Elastin Polypeptide (ME-ElaPoly)


2.2. Preparation of ME-ElaPoly/MFC Composite Gels by Photocrosslinking



2.3. Mechanical Properties of ME-ElaPoly/MFC Composite Gels



2.4. Cell Proliferation Assay


3. Experimental Section
3.1. Materials
3.2. Polymerization of a Fish Elastin Peptide (ElaPep)
3.3. Synthesis of a Methacrylate-Modified Fish Elastin Polypeptide (ME-ElaPoly)
3.4. Preparation of ME-ElaPoly/MFC Composite Gels by Photocrosslinking
3.5. Characterization
3.6. Cell Proliferation Assay
4. Conclusions
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W.; Parker, T.M. Mechanics of elastin: Molecular mechanism of biological elasticity and its relationship to contraction. J. Muscle Res. Cell Motil. 2003, 23, 543–559. [Google Scholar] [CrossRef]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef] [PubMed]
- Mithieux, S.M.; Rasko, J.E.J.; Weiss, A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials 2004, 25, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W.; Pattanaik, A.; Jie, X.; Cooper Woods, T.; McPherson, D.T.; Parker, T.M. Elastic protein-based polymers in soft tissue augmentation and generation. J. Biomater. Sci. Polym. Ed. 1998, 9, 1015–1048. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.A.; Conticello, V.P. Synthesis and characterization of elastin-mimetic protein gels derived from a well-defined polypeptide precursor. Macromolecules 2000, 33, 4809–4821. [Google Scholar] [CrossRef]
- Martino, M.; Perri, T.; Tamburro, A.M. Elastin-based biopolymers: Chemical synthesis and structural characterization of linear and cross-linked poly(OrnGlyGlyOrnGly). Biomacromolecules 2002, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, M.; Wadia, Y.; Hinds, M.T.; Teach, J.; Swartz, K.R.; Gregory, K.W. Successful repair of esophageal injury using an elastin based biomaterial patch. ASAIO J. 2001, 47, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Boughton, E.A.; Ruys, A.J.; Weiss, A.S.; Dehghani, F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 2009, 30, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Wolinsky, J.B.; Stone, P.J.; Wong, J.Y. Crosslinked α-elastin biomaterials: Towards a processable elastin mimetic scaffold. Acta Biomater. 2005, 1, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ε-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Betre, H.; Setton, L.A.; Meyer, D.E.; Chilkoti, A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 2002, 3, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Urban, J.P.G. The elastic network of articular cartilage: An immunohistochemical study of elastin fibres and microfibrils. J. Anat. 2010, 216, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Nakaba, M.; Ogawa, K.; Seiki, M.; Kunimoto, M. Properties of soluble elastin peptide from bulbus arteriosus in fish species. Fish. Sci. 2006, 72, 1322–1324. [Google Scholar] [CrossRef]
- Shiratsuchi, E.; Ura, M.; Nakaba, M.; Maeda, I.; Okamoto, K. Elastin peptides prepared from piscine and mammalian elastic tissues inhibit collagen-induced platelet aggregation and stimulate migration and proliferation of human skin fibroblasts. J. Pept. Sci. 2010, 16, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Marine structural proteins in biomedicine and tissue engineering. In Biological Materials of Marine Origin, Vertebrates; Springer: Dordrecht, The Netherlands, 2015; pp. 415–421. [Google Scholar]
- Teramoto, N.; Uchiumi, D.; Niikura, A.; Someya, Y.; Shibata, M. Polypeptide/layered silicate nanocomposites using fish-based collagen peptide: Effect of crosslinking and chain extension of the collagen peptide. J. Appl. Polym. Sci. 2007, 106, 4024–4030. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses and commercial potential. J. Appl. Polym. Sci.: Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from tempo-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. Hydrogels prepared from cross-linked nanofibrillated cellulose. ACS Sustain. Chem. Eng. 2014, 2, 772–780. [Google Scholar] [CrossRef]
- Borges, A.C.; Eyholzer, C.; Duc, F.; Bourban, P.-E.; Tingaut, P.; Zimmermann, T.; Pioletti, D.P.; Månson, J.-A.E. Nanofibrillated cellulose composite hydrogel for the replacement of the nucleus pulposus. Acta Biomater. 2011, 7, 3412–3421. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Johnstone, T.; Quinn, T.M.; Gray, D.G. Reinforcement with cellulose nanocrystals of poly(vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 2011, 7, 2373–2379. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interf. Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C.; Xie, X.-M. Studies on the properties and formation mechanism of flexible nanocomposite hydrogels from cellulose nanocrystals and poly(acrylic acid). J. Mater. Chem. 2012, 22, 22467–22480. [Google Scholar] [CrossRef]
- Karaaslan, M.A.; Tshabalala, M.A.; Yelle, D.J.; Buschle-Diller, G. Nanoreinforced biocompatible hydrogels from wood hemicelluloses and cellulose whiskers. Carbohydr. Polym. 2011, 86, 192–201. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.A.; Pereira, A.B.; Fajardo, A.R.; Rubira, A.; Muniz, E. Superabsorbent hydrogel nanocomposites based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Handa, K.; Nakagaito, A.N.; Yano, H. Optically transparent bionanofiber composites with low sensitivity to refractive index of the polymer matrix. Appl. Phys. Lett. 2005, 87, 243110. [Google Scholar] [CrossRef] [Green Version]
- Viet, D.; Beck-Candanedo, S.; Gray, D. Dispersion of cellulose nanocrystals in polar organic solvents. Cellulose 2007, 14, 109–113. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.S.; Lee, Y.M. Interpenetrating polymer network hydrogels based on poly(ethylene glycol) macromer and chitosan. Carbohydr. Polym. 2000, 41, 197–205. [Google Scholar] [CrossRef]
- Lopérgolo, L.C.; Lugão, A.B.; Catalani, L.H. Direct UV photocrosslinking of poly(N-vinyl-2-pyrrolidone) (pvp) to produce hydrogels. Polymer 2003, 44, 6217–6222. [Google Scholar] [CrossRef]
- Pan, J.-F.; Liu, N.-H.; Sun, H.; Xu, F. Preparation and characterization of electrospun plcl/poloxamer nanofibers and dextran/gelatin hydrogels for skin tissue engineering. PLoS One 2014, 9, e112885. [Google Scholar] [CrossRef] [PubMed]
- Leloup, V.M.; Colonna, P.; Buleon, A. Influence of amylose-amylopectin ratio on gel properties. J. Cereal Sci. 1991, 13, 1–13. [Google Scholar] [CrossRef]
- Ouchi, T.; Ichimura, S.; Ohya, Y. Synthesis of branched poly(lactide) using polyglycidol and thermal, mechanical properties of its solution-cast film. Polymer 2006, 47, 429–434. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Mechanical properties of the human Achilles tendon. Clin. Biomech. 2001, 16, 245–251. [Google Scholar] [CrossRef]
- Neumann, P.; Keller, T.S.; Ekström, L.; Perry, L.; Hansson, T.H.; Spengler, D.M. Mechanical properties of the human lumbar anterior longitudinal ligament. J. Biomech. 1992, 25, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.M.; Bae, W.C.; Chen, M.Q.; Lotz, M.; Amiel, D.; Coutts, R.D.; Sah, R.L. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthr. Cartil. 2007, 15, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.S.; van Osdol, W.W.; Dauskardt, R.H. Mechanical properties of human stratum corneum: Effects of temperature, hydration, and chemical treatment. Biomaterials 2006, 27, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, N.; Hayashi, A.; Yamanaka, K.; Sakiyama, A.; Nakano, A.; Shibata, M. Preparation and mechanical properties of photo-crosslinked fish gelatin/imogolite nanofiber composite hydrogel. Materials 2012, 5, 2573–2585. [Google Scholar] [CrossRef]
- Grinnell, F.; Feld, M.K. Initial adhesion of human fibroblasts in serum-free medium: Possible role of secreted fibronectin. Cell 1979, 17, 117–129. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, S.; Mori, M.; Teramoto, N.; Iisaka, M.; Suzuki, N.; Noto, M.; Kaimoto, Y.; Kakimoto, M.; Yamada, M.; Shiratsuchi, E.; et al. Preparation of Photocrosslinked Fish Elastin Polypeptide/Microfibrillated Cellulose Composite Gels with Elastic Properties for Biomaterial Applications. Mar. Drugs 2015, 13, 338-353. https://doi.org/10.3390/md13010338
Yano S, Mori M, Teramoto N, Iisaka M, Suzuki N, Noto M, Kaimoto Y, Kakimoto M, Yamada M, Shiratsuchi E, et al. Preparation of Photocrosslinked Fish Elastin Polypeptide/Microfibrillated Cellulose Composite Gels with Elastic Properties for Biomaterial Applications. Marine Drugs. 2015; 13(1):338-353. https://doi.org/10.3390/md13010338
Chicago/Turabian StyleYano, Shinya, Megumi Mori, Naozumi Teramoto, Makoto Iisaka, Natsumi Suzuki, Masanari Noto, Yasuko Kaimoto, Masashi Kakimoto, Michio Yamada, Eri Shiratsuchi, and et al. 2015. "Preparation of Photocrosslinked Fish Elastin Polypeptide/Microfibrillated Cellulose Composite Gels with Elastic Properties for Biomaterial Applications" Marine Drugs 13, no. 1: 338-353. https://doi.org/10.3390/md13010338
APA StyleYano, S., Mori, M., Teramoto, N., Iisaka, M., Suzuki, N., Noto, M., Kaimoto, Y., Kakimoto, M., Yamada, M., Shiratsuchi, E., Shimasaki, T., & Shibata, M. (2015). Preparation of Photocrosslinked Fish Elastin Polypeptide/Microfibrillated Cellulose Composite Gels with Elastic Properties for Biomaterial Applications. Marine Drugs, 13(1), 338-353. https://doi.org/10.3390/md13010338