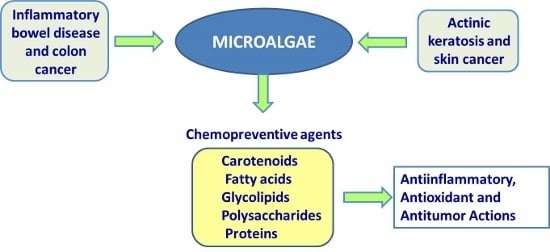
Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer
Abstract
:1. Introduction
2. Microalgae as a Source of Bioactive Molecules
| Compound | Source | Activity | References |
|---|---|---|---|
| CAROTENOIDS | |||
| β-Carotene | Dunaliella salina Haematococcus sp. | Antioxidant Pro-vitamin A Anti-inflammatory Anticancer | [30,37,38] |
| Astaxanthin | Haematococcus pluvialis Chlorella zofigiensis Chlorococcum sp. | Antioxidant Anti-inflammatory Anticancer | [39,40] |
| Lutein | Dunaliella salina Chlorella sorokiniana Chlorella prothecoides | Antioxidant Anti-inflammatory Anticancer | [41,42,43] |
| Violaxanthin | Dunaliella tertiolecta Chlorella ellipsoidea | Anti-inflammatory Anticancer | [44,45] |
| Zeaxanthin | Synechocystis sp. Chlorella saccharophila | Antioxidant Anti-inflammatory | [46,47] |
| Fucoxanthin | Phaeodactylum tricornutum Isochrysis sp. | Anticancer | [48,49] |
| FATTY ACIDS | |||
| Eicosapentaenoic acid (EPA) | Tetraselmis sp. | Antiinflammatory Anti-angiogenic | [50,51] |
| Docosahexaenic acid (DHA) | Tetraselmis sp. | Antiinflammatory Anti-angiogenic | [52,53] |
| Docosapentaenoic acid (DPA) | Nannochloropsis oculata | Antiinflammatory | [52,54] |
| GLYCOLIPIDS | |||
| Monogalactosyldiacylglycerol (MGDG) | Gymnodinium mikimotoi Stephanodiscus sp Pavlova lutheri Stephanodiscus sp. | Anticancer Antioxidant | [55,56] |
| Digalactosyldiacylglycerol (DGDG) | Stephanodiscus sp | Anticancer Antioxidant | [57,58] |
| Sulfo-quinovosyl-acyl-glycerol (SQAG) | Stephanodiscus sp | Anticancer Antioxidant | [57,59] |
| POLYSACCHARIDES | |||
| Sulphated extracellular polysaccharide | Diatom Phaeodactylum tricornutum | Anti-inflammatory Inmunomodulating | [60] |
| Sulphated polysaccharide Β-(1,3)-glucan | Chlorophyte Chlorella stigmatophora Chlorella vulgaris | Anti-inflammatory Inmunomodulating Anticancer | [60,61] |
| Sulphated polysaccharide | Prasinophyte Tetraselmis suecica | Anti-inflammatory | [62] |
| Sulphated polysaccharide | Haptophyte Isochrysis galbana | Anticancer | [63] |
| Sulphated polysaccharide | Rhodophyte Porphydium sp. | Anti-inflammatory Inmunomodulating Anticancer | [64] |
| Sulphated polysaccharide | Dinoflagellate Gyrodinium impudicum | Anti-inflammatory Inmunomodulating Anticancer | [65] |
| Extracellular polysaccharide s-Spirulan | Cyanobacteria Arthrospira platensis | Anticancer | [66] |
| PROTEIN AND PEPTIDES | |||
| Phycobiliproteins | Spirulina platensisPorphyridium sp. | Antioxidant Anti-inflammatory Anticancer | [67,68] |
| Peptides | Chlorella pyrenoidosa Cyanobacteria | Antioxidant Anti-inflammatory Anticancer | [69,70] |
| OTHER COMPOUNDS | |||
| Amides | Lyngbya majuscule | Anticancer | [71] |
| Quinones | Calothrix sp. | Anticancer | [72] |
| Phenolic compounds | Spirulina maxima Chlorella ellipsoidea Nannochloropsis sp | Antioxidant | [73,74] |
| Tocopherols | Porphydium sp. | Antioxidant | [75] |
3. Colorectal Cancer as a Consequence of Chronic Inflammatory Disorder
3.1. Molecular Pathways of Colon Carcinogenesis

3.2. Microalgae as a Source of Biomolecules with Potential in IBD and Colon Cancer
3.2.1. Carotenoids
3.2.1.1. β-Carotene
3.2.1.2. Astaxanthin
3.2.1.3. Lutein
3.2.1.4. Violaxanthin
3.2.1.5. Zeaxanthin
3.2.1.6. Fucoxanthin
3.2.2. Fatty Acids

3.2.3. Glycolipids
3.2.4. Polysaccharides
3.2.5. Proteins and Peptides
3.2.6. Other Compounds
4. Inflammation and Skin Cancer
4.1. Molecular Pathways of Skin Carcinogenesis
4.2. Microalgae as a Source of Biomolecules with Potential in Inflammation and Skin Cancer
4.2.1. Carotenoids
4.2.1.1. β-Carotene
4.2.1.2. Astaxanthin
4.2.1.3. Lutein
4.2.1.4. Zeaxanthin
4.2.1.5. Fucoxanthin
4.2.2. Fatty Acids
4.2.3. Glycolipids
4.2.4. Polyssacharides
4.2.5. Proteins
4.2.6. Other Compounds
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kansu, E. ISH 2014 World Congress: Report of the Chair of Council. Hematology 2014, 1, 433–434. [Google Scholar]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sue, S.; Shibata, W.; Maeda, S. Helicobacter pylori-induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis. Biomed. Res. Int. 2015, 2015, 737621. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Cheng, X.; Li, X.C.; Liu, Y.Q.; Wang, X.Q.; Shi, X.; Wang, Z.Y.; Guo, Y.Q.; Wen, Z.S.; Huang, Y.C.; et al. Tobacco smoke induces production of chemokine CCL20 to promote lung cancer. Cancer Lett. 2015, 363, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Ultraviolet radiation-induced inflammation activates β-catenin signaling in mouse skin and skin tumors. Int. J. Oncol. 2014, 44, 1199–1206. [Google Scholar] [PubMed]
- Malhotra, P.; Anwar, M.; Nanda, N.; Kochhar, R.; Wig, J.D.; Vaiphei, K.; Mahmood, S. Alterations in K-ras, APC and p53-multiple genetic pathway in colorectal cancer among Indians. Tumour Biol. 2013, 34, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Karahan, B.; Argon, A.; Yıldırım, M.; Vardar, E. Relationship between MLH-1, MSH-2, PMS-2,MSH-6 expression and clinicopathological features in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 4044–4053. [Google Scholar] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Motilva, V.; García-Mauriño, S.; Talero, E.; Illanes, M. New paradigms in chronic intestinal inflammation and colon cancer: Role of melatonin. J. Pineal Res. 2011, 51, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Arun, P.; Friedman, J.; Chen, Z.; van Waes, C. Current and potential inflammation targeted therapies in head and neck cancer. Curr. Opin. Pharmacol. 2009, 9, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sporn, B. The Big C for chemoprevention. Nature 2011, 471, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Gravitz, L. First Line of defence. Nature 2011, 471, S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Pikarsky, E.; Karin, M.; Coussens, L.M.; Chen, Y.C.; El-Omar, E.M.; Trinchieri, G.; Dubinett, S.M.; Mao, J.T.; Szabo, E.; et al. Cancer and inflammation: Promise for biologic therapy. J. Immunother. 2010, 33, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A. Statins and the colorectum: Hope for chemoprevention? Cancer Prev. Res. 2010, 3, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.E. The search for a chemoprevention agent effective against melanoma: Considerations and challenges. J. Investig. Dermatol. 2011, 131, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Metformin and other biguanides in oncology: Advancing the research agenda. Cancer Prev. Res. 2010, 3, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; George, J. Combinatorial strategies employing nutraceuticals for cancer development. Ann. N. Y. Acad. Sci. 2011, 1229, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [PubMed]
- Tartar, A.; Boucias, D.G.; Becnel, J.J.; Adams, B.J. Comparison of plastid 16S rRNA (rrn16) genes from Helicosporidium spp.: Evidence supporting the reclassification of Helicosporidia as green algae (Chlorophyta). Int. J. Syst. Evol. Microbiol. 2003, 53, 1719–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, R.; Urano, N.; Suzuki, M. Phylogeny of the non photosynthetic green micro-algal genus Prototheca (Trebouxiophyceae, Chlorophyta) and related taxa inferred from SSU and LSU ribosomal DNA partial sequence data. FEMS Microbiol. Lett. 2003, 223, 275–280. [Google Scholar] [CrossRef]
- Tartar, A.; Boucias, D.G.; Adams, B.J.; Becnel, J.J. Phylogenetic analysis identifies the invertebrate pathogen Helicosporidium sp. as a green alga (Chlorophyta). Int. J. Syst. Evol. Microbiol. 2002, 52, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irigoien, X.; Huisman, J.; Harris, R.P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 2004, 429, 863–867. [Google Scholar] [CrossRef] [PubMed]
- López-Urrutia, A.; San Martin, E.; Harris, R.P.; Irigoien, X. Scaling the metabolic balance of the oceans. Proc. Natl. Acad. Sci. USA 2006, 6, 8739–8744. [Google Scholar] [CrossRef] [PubMed]
- Raff, J.D.; Njegic, B.; Chang, W.L.; Gordon, M.S.; Dabdub, D.; Gerber, R.B.; Finlayson-Pitts, B.J. Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride. Proc. Natl. Acad. Sci. USA 2009, 106, 13647–13654. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Ferreira, M.; Coutinho, P.; Fábregas, J.; Otero, A. Delivery of astaxanthin from Haematococcus pluvialis to the aquaculture food chain. Aquaculture 2005, 250, 424–430. [Google Scholar] [CrossRef]
- Clarens, A.; Resurreccion, E.; White, M.; Colosi, L. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Norsker, N.; Barbosa, M.; Vermue, M.; Wijffels, R. Microalgal production-a close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Iconomou, D.; Sotiroudis, T.; Israilides, C.; Muylaert, K. Exploration of using stripped ammonia and ash from poultry litter for the cultivation of the cyanobacterium Arthrospira platensis and the green microalga Chlorella vulgaris. Bioresour. Technol. 2015, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kilian, O.; Benemann, C.; Niyogi, K.; Vick, B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Sharma, A.K.; Daniell, H.; Kumar, S. Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2. Plant Biotechnol. J. 2015, 13, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.K.; Danielewicz, M.A.; Wong, D.M.; Anderson, L.A.; Boothe, J.R. Phenotypic screening with oleaginous microalgae reveals modulators of lipid productivity. ACS Chem. Biol. 2013, 8, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Schenk, P.M. Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnol. Bioeng. 2015, 112, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Polle, J.J.; Tran, D.; Cushman, J.C.; Jin, E.; Varela, J.C. The unicellular green alga Dunaliella salina Teod. as a model for abiotic stress tolerance: Genetic advances and future perspectives. Algae 2011, 26, 3–20. [Google Scholar] [CrossRef]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of b-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Gudmundsson, O.; Paglia, G.; Herjolfsson, G.; Andrésson, O.S.; Palsson, B.Ø.; Brynjolfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodríguez, H. Enhancement of lutein production in Chlorella sorokiniana (chlorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.M.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Beuf, L.; Vermaas, W. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2000, 66, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Puri, M.; Wilkens, S.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour. Technol. 2013, 143, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, J.H.; Kim, H. Beta-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-kappaB and IL-8 expression in gastric epithelial AGS cells. J. Nutr. Sci. Vitaminol. 2011, 57, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Toci, A.T.; Mangini, S.; Wrubl, F.; Rodolfi, L.; Tredici, M.R.; Coletta, A.; Antonacci, D. Determination of fucoxanthin isomers in microalgae (Isochrysis sp.) by high-performance liquid chromatography coupled with diode-array detector multistage mass spectrometry coupled with positive electrospray ionization. Rapid Commun. Mass Spectrom. 2013, 27, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Mobraten, K.; Haug, T.M.; Kleiveland, C.R.; Lea, T. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis. 2013, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Lim, D.K.; Schenk, P.M. Effects of long chain fatty acid synthesis and associated gene expression in microalga Tetraselmis sp. Mar. Drugs 2014, 12, 3381–3398. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.; Mann, C.; Metcalfe, M.; Webb, M.; Pollard, C.; Spencer, D.; Berry, D.; Steward, W.; Dennison, A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer 2009, 45, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Han, J.I. Hydrothermal-acid treatment for effectual extraction of eicosapentaenoic acid (EPA)-abundant lipids from Nannochloropsis salina. Bioresour. Technol. 2015, 191, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nauroth, J.M.; Liu, Y.C.; van Elswyk, M.; Bell, R.; Hall, E.B.; Chung, G.; Arterburn, L.M. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids 2010, 45, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Sahara, H.; Kumamoto-Yonezawa, Y.; Kuriyama, I.; Hada, T.; Sato, N.; Yoshida, H.; Mizushina, Y. Anti-tumor effect of orally administered spinach glycolipid fraction on implanted cancer cells, colon-26, in mice. Lipids 2008, 43, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Hada, T.; Yoshida, H. In vivo antitumor effect of liposomes with sialyl Lewis X including monogalactosyl diacylglycerol, a replicative DNA polymerase inhibitor, from spinach. Oncol. Rep. 2012, 28, 821–828. [Google Scholar] [PubMed]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of differentiation and apoptosis mediated by sodium butyrate in Caco-2 cells with algal glycolipids. In Vitro Cell Dev. Biol. Anim. 2005, 41, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory effects of preventive and curative orally administered spinach glycoglycerolipid fraction on the tumor growth of sarcoma and colon in mouse graft models. Food Chem. 2009, 112, 205–210. [Google Scholar] [CrossRef]
- Maeda, N.; Kokai, Y.; Hada, T.; Yoshida, H.; Mizushina, Y. Oral administration of monogalactosyl diacylglycerol from spinach inhibits colon tumor growth in mice. Exp. Ther. Med. 2013, 5, 17–22. [Google Scholar] [PubMed]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-Inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K.; Yokokura, T.; Satoh, H.; Mutai, M. Anti-tumor effect by oral administration of Chlorella extract, PCM-4 by oral admission. Gan To Kagaku Zasshi 1983, 10, 781–785. [Google Scholar]
- Jo, W.S.; Cho, Y.J.; Kim, H.J.; Nam, B.Y.; Hong, S.H.; Lee, G.A.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. Anti-inflammatory effect of microalgal extracts from Tetraselmis suecica. Food Sci. Biotechnol. 2010, 19, 1519–1528. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-d-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.M.; Muizzudin, N.; Arad, S.M.; Marenus, K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulphated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Challouf, R.; Trabelsi, L.; Dhieb, R.B.; El Abed, O.; Yahia, A.; Ghozzi, K.; Ammar, J.B.; Omran, H.; Ouada, H.B. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011, 54, 831–838. [Google Scholar] [CrossRef]
- Romay, Ch.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.H.; Wang, Y.J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Piplani, H.; Vaish, V.; Sanyal, S.N. Dolastatin 15, a mollusk linear peptide, and Celecoxib, a selective cyclooxygenase-2 inhibitor, prevent preneoplastic colonic lesions and induce apoptosis through inhibition of the regulatory transcription factor NF-κB and an inflammatory protein, iNOS. Eur. J. Cancer Prev. 2012, 21, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Hatae, N.; Satoh, R.; Chiba, H.; Osaki, T.; Nishiyama, T.; Ishikura, M.; Abe, T.; Hibino, S.; Choshi, T.; Okada, C.; et al. N-Substituted calothrixin B derivatives inhibited the proliferation of HL-60 promyelocytic leukemia cells. Med. Chem. Res. 2014, 23, 4956–4961. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El Baroty, G.S. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010, 90, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.S.; Chen, C.F.; Yee, W.; Aziz, A.; Loh, S.H. Cinnamic acid, coumarin and vanillin: Alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J. Microbiol. Methods 2011, 84, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; García-Martínez, D.; Ruperez, F.J.; Martín-Álvarez, P.J.; Reglero, G.; Cifuentes, A.; Barbas, C.; Ibañez, E.; Señoráns, F.J. Enrichment of vitamin E from Spirulina platensis microalga by SFE. J. Supercrit. Fluid. 2008, 43, 484–489. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Lapointe, T.K.; Beck, P.L.; Buret, A.G. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A. Recently identified and potential targets for colon cancer treatment. Future Oncol. 2010, 6, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A.; Illanes, M.; Motilva, V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflamm. Bowel Dis. 2011, 17, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.H.; Goldman, H.; Ransohof, D.F.; Appelman, H.; Fenoglio, C.M.; Haggitt, R. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical application. Hum. Pathol. 1983, 14, 931–968. [Google Scholar]
- Pascal, R.R. Dysplasia and early carcinoma in inflammatory bowel disease and colorectal carcinomas. Hum. Pathol. 1994, 25, 1160–1171. [Google Scholar] [CrossRef]
- Bird, P.R.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxico. Lett. 2000, 112, 295–402. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.; Kido, K.; Yoshitake, H.; Flanigan, A. Dysplasia and cancer in the dextran sulphate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: A study of histopathology, β-catenin and expression and the role of inflammation. Carcinogenesis 2000, 21, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Andersson, M.; Lagerstedt, K.; Lönnroth, C.; Nordgren, S.; Lundholm, K. Receptor and enzyme expression for prostanoid metabolism in colorectal cancer related to tumor tissue PGE2. Int. J. Oncol. 2010, 36, 469–478. [Google Scholar] [PubMed]
- Rao, S.K.; Pavicevic, Z.; Du, Z.; Kim, J.G.; Fan, M.; Jiao, Y.; Rosebush, M.; Samant, S.; Gu, W.; Pfeffer, L.M.; et al. Pro-inflammatory genes as biomarkers and therapeutic targets in oral squamous cell carcinoma. J. Biol. Chem. 2010, 285, 32512–32521. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; López-Pedrera, C.; Haba-Rodríguez, J.R.; Rodríguez-Ariza, A. Nitric oxide and cancer: The emerging role of S-nitrosylation. Curr. Mol. Med. 2012, 12, 50–67. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Role of oxygen in cancer: Looking beyond hypoxia. Anticancer Agents Med. Chem. 2009, 9, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.A.; Chiang, B.L. Inflammasomes and human autoimmunity: A comprehensive review. J. Autoimmun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Shim, D.W.; Shin, W.Y.; Heo, K.H.; Kwak, S.B.; Sim, E.J.; Jeong, J.H.; Kang, T.B.; Lee, K.H. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation. Int. J. Mol. Sci. 2015, 16, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, D.I.; Kim, S.H.; Lee, H.; Lee, K.S.; Cho, S.H.; Lee, Y.C. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014, 5, e1498. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Actis, G.C. The gut microbiome. Inflamm. Allergy Drug Targets 2014, 13, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Kamada, N.; Hisamatsu, T. Clinical strategies for the blockade of IL-18 in inflammatory bowel diseases. Curr. Drug Targets 2013, 14, 1392–1329. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Núñez, G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology 2011, 141, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Vogel, P.; Body-Malapel, M.; Lamkanfi, M.; Kanneganti, T.D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 2010, 185, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.M.; Wang, E.; Ma, W.; Haines, D.; O’hUigin, C.; et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: Role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Elinav, E.; Flavell, R.A. Inflammasome-mediated suppression of inflammation-induced colorectal cancer progression is mediated by direct regulation of epithelial cell proliferation. Cell Cycle 2011, 10, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; TeKippe, E.M.; Woodford, R.M.; Uronis, J.M.; Holl, E.K.; Rogers, A.B.; Herfarth, H.H.; Jobin, C.; Ting, J.P. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010, 207, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Leeth, R.A.; Rothschild, D.E.; Coutermarsh-Ott, S.L.; McDaniel, D.K.; Simmons, A.E.; Heid, B.; Cecere, T.E.; Allen, I.C. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J. Immunol. 2015, 194, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Cardus, A.; Uryga, A.K.; Walters, G.; Erusalimsky, J.D. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc. Res. 2013, 97, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- García-Mauriño, S.; Alcaide, A.; Domínguez, C. Pharmacological control ofautophagy: Therapeutic perspectives in inflammatory bowel disease and colorectal cancer. Curr. Pharm. Des. 2012, 18, 3853–3873. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ogura, T.; Kishimoto, A.; Minegishi, Y.; Nakajima, N.; Miyazaki, M.; Esumi, H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 2002, 21, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, B.S.; Kitayama, J.; Nagawa, H. Adiponectin supports cell survival in glucose deprivation through enhancement of autophagic response in colorectal cancer cells. Cancer Sci. 2011, 102, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Ko, S.H.; Kim, J.S. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 2014, 29, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Blander, G.; Michan, S.; Oberdoerffer, P.; Ogino, S.; Campbell, J.; Bhimavarapu, A.; Luikenhuis, S.; de Cabo, R.; Fuchs, C.; et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 2008, 16, e2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Sengupta, K.; Li, C.; Kim, H.S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008, 14, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Kabra, N.; Li, Z.; Chen, L.; Li, B.; Zhang, X.; Wang, C.; Yeatman, T.; Coppola, D.; Chen, J. Sirt1 is an inhibitor of proliferation and tumor formation in colon cancer. J. Biol. Chem. 2009, 284, 18210–18217. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Min, K.W.; Paik, S.S.; Jang, K.S. Loss of SIRT1 histone deacetylase expression associates with tumour progression in colorectal adenocarcinoma. J. Clin. Pathol. 2012, 65, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Kriegl, L.; Vieth, M.; Kirchner, T.; Menssen, A. Up-regulation of c-MYC and SIRT1 expression correlates with malignant transformation in the serrated route to colorectal cancer. Oncotarget 2012, 3, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Holloway, K.R.; Calhoun, T.N.; Saxena, M.; Metoyer, C.F.; Kandler, E.F.; Rivera, C.A.; Pruitt, K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 9216–9221. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.P.; Zhong, L.; Mostoslavsky, R. The histone deacetylase SIRT6: At the crossroads between epigenetics, metabolism and disease. Curr. Top Med. Chem. 2013, 13, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I. Carotenoids in nature: Insights from plants and beyond. Func. Plant Biol. 2011, 38, 833–847. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–64. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Eonseon, J.; Polle, J.E.W.; Lee, H.K.; Hyund, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. Microb. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Brown, M.R. Nutritional value of microalgae for aquaculture. In Advances en Nutrición Acuicola VI. Memorias del VI Simposium Internacional de Nutrición Acuicola, Cancun, Quintana Roo, Mexico 3–6 September 2002; Cruz-Suárez, L.E., Ricque-Marie, D., Tapia-Salazar, M., Gaxiola-Cortés, M.G., Simoes, N., Eds.; Universidad Autónoma de Nuevo León: Monterrey, N.L., México; p. 282.
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Saba, N. Retinoids as chemoprevention for head and neck cancer: Where do we go from here? Crit. Rev. Oncol. Hematol. 2005, 55, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Lotan, R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 2002, 41, 41–55. [Google Scholar] [CrossRef]
- Lotan, R. Retinoids and apoptosis: Implications for cancer chemoprevention and therapy. J. Natl. Cancer Inst. 1995, 87, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Mokady, S.; Avron, M. The beta-carotene-rich alga Dunaliella bardawil as a source of retinol in a rat diet. Br. J. Nutr. 1988, 59, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Wedick, N.M.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B. Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am. J. Clin. Nutr. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; Sinha, R.; Petersen, G.M. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J. Gastrointest. Cancer 2013, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Serchi, T.; Renaut, J.; Hoffmann, L.; Bohn, T. Carotenoid exposure of Caco-2 intestinal epithelial cells did not affect selected inflammatory markers but altered their proteomic response. Br. J. Nutr. 2012, 108, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.P.; Jena, G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2015, 54, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Lavy, A.; Naveh, Y.; Coleman, R.; Mokady, S.; Werman, M.J. Dietary Dunaliella bardawil, a beta-carotene-rich alga, protects against acetic acid-induced small bowel inflammation in rats. Inflamm. Bowel Dis. 2003, 9, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Rumi, G., Jr.; Szabo, I.; Vincze, A.; Matus, Z.; Tóth, G.; Mózsik, G. Decrease of serum carotenoids in Crohn’s disease. J. Physiol. Paris 2000, 94, 159–161. [Google Scholar] [CrossRef]
- Wendland, B.E.; Aghdassi, E.; Tam, C.; Carrrier, J.; Steinhart, A.H.; Wolman, S.L.; Baron, D.; Allard, J.P. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am. J. Clin. Nutr. 2001, 74, 259–264. [Google Scholar] [PubMed]
- Geerling, B.J.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.J. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur. J. Clin. Nutr. 2000, 54, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Calviello, G. Beta-carotene downregulates the steady-state and heregulin-alpha-induced COX-2 pathways in colon cancer cells. J. Nutr. 2005, 135, 129–136. [Google Scholar] [PubMed]
- Choi, S.Y.; Park, J.H.; Kim, J.S.; Kim, M.K.; Aruoma, O.I.; Sung, M.K. Effects of quercetin and beta-carotene supplementation on azoxymethane-induced colon carcinogenesis and inflammatory responses in rats fed with high-fat diet rich in omega-6 fatty acids. Biofactors 2006, 27, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.N.T.; Leclerc, D.; Levesque, N.; Deng, L.; Rozen, R. β,β-Carotene 15,15′-monooxygenase and its substrate β-carotene modulate migration and invasion in colorectal carcinoma cells. Am. J. Clin. Nutr. 2013, 98, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Raju, J.; Swamy, M.V.; Cooma, I.; Patlolla, J.M.; Pittman, B.; Reddy, B.S.; Steele, V.E.; Rao, C.V. Low doses of beta-carotene and lutein inhibit AOM-induced rat colonic ACF formation but high doses augment ACF incidence. Int. J. Cancer 2005, 113, 798–802. [Google Scholar]
- Mayne, S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996, 10, 690–701. [Google Scholar] [PubMed]
- Mänistö, S.; Yaun, S.S.; Hunter, D.J.; Spiegelman, D.; Adami, H.O.; Albanes, D.; van den Brandt, P.A.; Buring, J.E.; Cerhan, J.R.; Colditz, G.A.; et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am. J. Epidemiol. 2007, 165, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Joshi, A.M.; Ohnaka, K.; Morita, M.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; et al. Dietary Intakes of Retinol, Carotenes, Vitamin C, and Vitamin E and Colorectal Cancer Risk: The Fukuoka Colorectal Cancer Study. Nutr. Cancer 2012, 64, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Leufkens, A.M.; Siersema, P.D.; van Duijnhoven, F.J.; Vrieling, A.; Hulshof, P.J.; van Gils, C.H.; Overvad, K.; Roswall, N.; Kyrø, C.; et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2014, 135, 2930–2939. [Google Scholar]
- Brown, G.T.; Cash, B.G.; Blihoghe, D.; Johansson, P.; Alnabulsi, A.; Murray, G.I. The expression and prognostic significance of retinoic acid metabolising enzymes in colorectal cancer. PLoS ONE 2014, 9, e90776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropotova, E.S.; Zinovieva, O.L.; Zyryanova, A.F.; Dybovaya, V.I.; Prasolov, V.S.; Beresten, S.F.; Oparina, N.Y.; Mashkova, T.D. Altered expression of multiple genes involved in retinoic acid biosynthesis in human colorectal cancer. Pathol. Oncol. Res. 2014, 20, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Chaiter, Y.; Gruber, S.B.; Ben-Amotz, A.; Almog, R.; Rennert, H.S.; Fischler, R.; Rozen, G.; Rennert, G. Smoking attenuates the negative association between carotenoids consumption and colorectal cancer risk. Cancer Causes Control 2009, 20, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Kim, M.Y.; Sarto, G.E.; Shikany, J.M.; Rohan, T.E. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. Eur. J. Clin. Nutr. 2012, 66, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Wu, K.; Giovannucci, E.; Spiegelman, D.; Willett, W.C.; Smith-Warner, S.A. Carotenoid intake and risk of colorectal adenomas in a cohort of male health professionals. Cancer Causes Control 2013, 24, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Fang, Y.J.; Chen, Y.M.; Luo, W.P.; Pan, Z.Z.; Zhong, X.; Zhang, C.X. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: A case-control study. Eur. J. Nutr. 2015, 54, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Gross, M. Astaxanthin, a carotenoid without vitamin A activity, augments antibody responses in cultures including T-helper cell clones and suboptimal doses of antigen. J. Nutr. 1995, 125, 2483–2492. [Google Scholar] [PubMed]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoidastaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–317. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Park, Y.S.; Choi, D.K.; Chang, H.I. Effects of astaxanthin on the production of NO and the epression of COX-2 and iNOS in LPS-simulated BV2 microglial cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [PubMed]
- Tanaka, T.; Kawamori, T.; Ohnishi, M.; Makita, H.; Mori, H.; Satoh, K.; Hara, A. Suppression of azomethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis 1995, 16, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.N.; Ashokkumar, P.; Sudhandiran, G. Antioxidative and antiproliferative effects of astaxanthin during the initiation stages of 1,2-dimethyl hydrazineinduced experimental colon carcinogenesis. Fund. Clin. Pharmacol. 2009, 23, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. (Lond.) 2010, 5, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, H.H.; Cho, S.; Lee, S.R.; Shin, M.H.; Chung, J.H. Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sevilla, J.M.; Acien Fernandez, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, S.; Knaus, M.; Suh, M. Associations between lutein, zeaxanthin, and age-related macular degeneration: An overview. Crit. Rev. Food Sci. Nutr. 2009, 49, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Sharavana, G.; Ramaprasada, T.R.; Vallikannan, B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. 2015, 6, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Ma, K.N.; Caan, B.J.; Sweeney, C.; Wolff, R.; Samowitz, W.S.; Potter, J.D.; Slattery, M.L. Interactions of peroxisome proliferator-activated receptor {gamma} and diet in etiology of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Loarca-Piña, G.F.; Salgado, L.M.; Ramos-Gomez, M. Dietary supplementation of lutein reduces colon carcinogenesis in DMH-treated rats by modulating K-ras, PKB, and β-catenin proteins. Nutr. Cancer 2011, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, H.B.; Wong, R.N.; Ji, B.; Jiang, Y. Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J. Chromatogr. A. 2005, 1064, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Hong, S.E.; Wang, Y.C.; Hsu, S.L.; Hsiang, D.; Chang, C.M. Preparation of highly pure zeaxanthin particles from sea water-cultivated microalgae using supercritical anti-solvent recrystallization. Bioresour. Technol. 2012, 104, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Qin, T.; Ren, Z.; Wu, D.; Shang, F. Lutein or zeaxanthin supplementation suppresses inflammatory responses in retinal pigment epithelial cells and macrophages. Adv. Exp. Med. Biol. 2012, 723, 43–50. [Google Scholar] [PubMed]
- Okuyama, Y.; Ozasa, K.; Oki, K.; Nishino, H.; Fujimoto, S.; Watanabe, Y. Inverse associations between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm in Japanese. Int. J. Clin. Oncol. 2014, 19, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Gagez, A.L.; Thiery, V.; Pasquet, V.; Cadoret, J.P.; Picot, L. Epoxycarotenoids and cancer. Review. Curr. Bioact. Compd. 2012, 8, 109–141. [Google Scholar] [CrossRef]
- Maeda, H.; Masashi Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, S.M. Application of microalgal fucoxanthin for the reduction of colon cancer risk: Inhibitory activity of fucoxanthin against beta-glucuronidase and DLD-1 cancer cells. Nat. Prod. Commun. 2014, 9, 921–924. [Google Scholar] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, K.; Michaud, D.S.; Rimm, E.B.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 64–67. [Google Scholar] [PubMed]
- Cheng, J.; Ogawa, K.; Kuriki, K.; Yokoyama, Y.; Kamiya, T.; Seno, K.; Okuyama, H.; Wang, J.; Luo, C.; Fujii, T.; et al. Increased intake of n-3 poly (DPA)unsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003, 193, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dommels, Y.E.; Haring, M.M.; Keestra, N.G.; Alink, G.M.; van Bladeren, P.J.; van Ommen, B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis 2003, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Mittmann, U.; Bar-Meir, S.; D’Haens, G.; Bradette, M.; Cohen, A.; Dallaire, C.; Ponich, T.P.; McDonald, J.W.; et al. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: The EPIC Randomized Controlled Trials. JAMA 2008, 299, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Bizzarro, A.; Piccioni, E.; Fasano, E.; Rossi, C.; Lauria, A.; Cittadini, A.R.; Masullo, C.; Calviello, G. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer’s patients. Curr. Alzheimer Res. 2012, 9, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Can vagus nerve stimulation halt or ameliorate rheumatoid arthritis and lupus? Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Brahmbhatt, V.; Oliveira, M.; Martin, F.P.; Lichti, P.; Raymond, F.; Mansourian, R.; Metairon, S.; Pace-Asciak, C.; Bastic Schmid, V.; et al. Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Mobraten, K.; Haugbro, T.; Karlstrom, E.; Kleiveland, C.R.; Lea, T. Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J Recept Signal Transduct Res. 2014, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Beelen, V.A.; Spenkelink, B.; Mooibroek, H.; Sijtsma, L.; Bosch, D.; Rietjens, I.M.; Alink, G.M. An n-3 PUFA-rich microalgal oil diet protects to a similar extent as a fish oil-rich diet against AOM-induced colonic aberrant crypt foci in F344 rats. Food Chem. Toxicol. 2009, 47, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Kralovec, J.; Bailey-Hall, E.; Smeberg, V.; Stark, J.G.; Salem, N., Jr. Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 2015, 14, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Mbodji, K.; Hassan, A.; Aziz, M.; Boukhettala, N.; Coëffier, M.; Savoye, G.; Déchelotte, P.; Marion-Letellier, R. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin. Nutr. 2011, 30, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hoover, R.L.; Williams, J.D.; Sperling, R.I.; Ravalese, J., III; Spur, B.W.; Robinson, D.R.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Effects of dietary enrichment with eicosapentaenoic acid and docosahexaenoic acid on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Eng. J. Med. 1985, 312, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [PubMed]
- Sun, Y.P.; Tjonahen, E.; Keledjian, R.; Zhu, M.; Yang, R.; Recchiuti, A.; Pillai, P.S.; Petasis, N.A.; Serhan, C.N. Anti-inflammatory and pro-resolving properties of benzo-lipoxin A4 analogs. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- De Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; de la Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and their activity as TNF-α inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Avila-Román, J.; Talero, E.; Alcaide, A.; de los Reyes, C.; Zubía, E.; García-Mauriño, S.; Motilva, V. Preventive effect of the microalga Chlamydomonas debaryana on the acute phase of experimental colitis in rats. Br. J. Nutr. 2014, 112, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Gagliardi, C.; Vetro, M.; Ronchetti, F.; Takasaki, M.; Konoshima, T.; Suzuki, N.; Tokuda, H. New 6-amino-6-deoxy-glycoglycerolipids derived from 2-O-b-d-glu-copyranosylglycerol: Insights into the structure–activity relationship of glycoglycerolipids as anti-tumor promoters. Carbohydr. Res. 2013, 373, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, A.M.; Schwarting, G.A.; Robbins, P.W. Glycolipids of the mouse peritoneal macrophage. Alterations in amount and surface exposure of specific glycolipid species occur in response to inflammation and tumoricidal activation. J. Exp. Med. 1984, 160, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.C.; Bodennec, G.; Gentien, P. Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry 1998, 47, 783–787. [Google Scholar] [CrossRef]
- Meireles, L.A.; Guedes, A.C.; Malcata, F.X. Lipid class composition of the microalga Pavlova lutheri: Eicosapentaenoic and docosahexaenoic acids. J. Agric. Food Chem. 2003, 51, 2237–2241. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, D.; Yan, X.; Chen, J.; Zhou, C. Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight mass spectrometry. Anal. Chim. Acta 2010, 663, 60–68. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, K.M.; McNichol, J.; McGinn, P.J.; O’Leary, S.J.; Melanson, J.E. Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography-high-resolution mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Matsuyama, S.; Igarashi, K.; Utsumi, M.; Shiraiwa, Y.; Kuwabara, T. Anaerobic coculture of microalgae with Thermosipho globiformans and Methanocaldococcus jannaschii at 68 °C enhances generation of n-alkane-rich biofuels after pyrolysis. Appl. Environ. Microbiol. 2013, 79, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; O’Leary, S.J. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Hada, T.; Murakami-Nakai, C.; Kuriyama, I.; Ichikawa, H.; Fukumori, Y.; Hiratsuka, J.; Yoshida, H.; Sakaguchi, K.; Mizushina, Y. Effects of DNA polymerase inhibitory and antitumor activities of lipase-hydrolyzed glycolipid fractions from spinach. J. Nutr. Biochem. 2005, 16, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kagan, M.L.; Levy, A.; Leikin-Frenkel, A. Comparative study of tissue deposition of omega-3 fatty acids from polar-lipid rich oil of the microalgae Nannochloropsis oculata with krill oil in rats. Food Funct. 2015, 6, 186–192. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of polysaccharides from marine microalgae. In Polysaccharides: Bioactivity and Biotechnology; Merillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.P.; Sun, X.N.; Cui, L.J.; Cao, Q.F.; Zhuang, G.F.; Deng, T.Z.; Zhang, D.Y. Suppressive effect of pectic polysaccharides extracted from Rauwolfia verticillata (Lour.) Baill.var.hainanensis Tsiang on inflammation by regulation of NF-κB pathway and interleukin-17 in mice with dextran sulphatesodium-induced ulcerative colitis. Asian Pac. J. Trop. Med. 2015, 8, 147–152. [Google Scholar] [CrossRef]
- Hartog, A.; Belle, F.N.; Bastiaans, J.; de Graaff, P.; Garssen, J.; Harthoorn, L.F.; Vos, A.P. A potential role for regulatory T-cells in the amelioration of DSS induced colitis by dietary non-digestible polysaccharides. J. Nutr. Biochem. 2015, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Yanase, K.; Suzuki, M.; Okutani, K.; Yamori, T.; Andoh, T. Inhibition of DNA topoisomerases I and II, and growth inhibition of human cancer cell lines by a marine microalgal polysaccharide. Biochem. Pharm. 2003, 66, 481–487. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Pugh, N.D.; Ma, G.; Pasco, D.S. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice. Int Immunopharmacol. 2006, 6, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, O.; Ishii, K.; Kawamura, C.; YenHei, S.; YeBao, N.; Hirahashi, T.; Katoh, T. Enhancement of mucosal immune functions by dietary Spirulina plantensis in human and animals. Nutr. Sci. 2004, 7, 31–34. [Google Scholar]
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [PubMed]
- Li, B.; Gao, M.H.; Zhang, X.C.; Chu, X.M. Molecular immune mechanism of C-phycocyanin from Spirulina platensis induces apoptosis in HeLa cells in vitro. Biotechnol. Appl. Biochem. 2006, 43, 155–164. [Google Scholar]
- Saini, M.K.; Sanyal, S.N.; Vaiphei, K. Piroxicam and C-phycocyanin mediated apoptosis in 1,2-dimethylhydrazine dihydrochloride induced colon carcinogenesis: Exploring the mitochondrial pathway. Nutr. Cancer 2012, 64, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.K. Bioactive peptides from marine sources as potential anti-inflammatory therapeutics. Curr. Protein Pept. Sci. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Kim, S.K. Beneficial effect of peptides from microalgae on anticancer. Curr. Protein Pept. Sci. 2013, 14, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Chen, J.R. Identification of antihypertensive peptides from peptic digests of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wrasidlo, W.; Mielgo, A.; Torres, V.A.; Barbero, S.; Stoletov, K.; Suyama, T.L.; Klemke, R.L.; Gerwick, W.H.; Carson, D.A.; Stupack, D.G. The marine lipopeptide somocystinamide A triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA 2008, 105, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a potent cytotoxic cyclodepsipeptide from papua new guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Ross, C.; Paul, V.J.; Matthew, S.; Luesch, H. Dragonamides C and D, linear lipopeptides from the marine cyanobacterium brown Lyngbya polychroa. J. Nat. Prod. 2008, 71, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Andrianasolo, E.H.; Goeger, D.; Gerwick, W.H. Mitsoamide: A cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl. Chem. 2007, 79, 593–602. [Google Scholar] [CrossRef]
- Chaganty, S.; Golakoti, T.; Heltzel, C.; Moore, R.E.; Yoshida, W.Y. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp. GSV 224. J. Nat. Prod. 2004, 67, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Nakeff, A.E.; Media, J.E.; Wiegand, R.A.; Valeriote, F.A. Inhibition of macromolecular synthesis by cryptophycin-52. Anticancer Drugs 2002, 13, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, S.L.; Leal, R.M.; Tinley, T.L.; Luesch, H.; Moore, R.E.; Corbett, T.H. The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int. J. Cancer 2003, 104, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Kale, A.J.; Fenley, A.T.; Byrum, T.; Debonsi, H.M.; Gilson, M.K.; Valeriote, F.A.; Moore, B.S.; Gerwick, W.H. The carmaphycins: New proteasome inhibitors exhibiting an α,β-epoxyketone warhead from a marine cyanobacterium. Chembiochem 2012, 13, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Krunic, A.; Shen, Q.; Swanson, S.M.; Orjala, J. Minutissamides A–D, antiproliferative cyclic decapeptides from the cultured cyanobacterium Anabaena minutissima. J. Nat. Prod. 2011, 74, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C–F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Wang, Z.; Xing, X.; Maguire, A. R.; Luesch, H.; Zhang, H.; Xu, Z.; Ye, T. Total synthesis and biological evaluation of Grassypeptolide A. Chem. Eur. J. 2013, 19, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Fennell, B.J.; Carolan, S.; Pettit, G.R.; Bell, A. Effects of the antimitotic natural product dolastatin 10, and related peptides, on the human malarial parasite Plasmodium falciparum. J. Antimicrob. Chemother. 2003, 51, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Aherne, G.W.; Hardcastle, A.; Valenti, M.; Bryant, A.; Rogers, P.; Pettit, G.R.; Srirangam, J.K.; Kelland, L.R. Antitumour evaluation of dolastatins 10 and 15 and their measurement in plasma by radioimmunoassay. Cancer Chemother. Pharmacol. 1996, 38, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Natsume, T.; Tamaoki, S.; Watanabe, J.; Asano, H.; Mikami, T.; Miyasaka, K.; Miyazaki, K.; Gondo, M.; Sakakibara, K.; et al. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Jpn. J. Cancer Res. 1997, 88, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Fujita, F.; Koike, M.; Fujita, M.; Sakamoto, Y.; Tsukagoshi, S. Antitumor effects of TZT-1027, a novel dolastatin 10 derivative, on human tumor xenografts in nude mice. Gan To Kagaku Ryoho. 2000, 27, 451–458. [Google Scholar] [PubMed]
- Shnyder, S.D.; Cooper, P.A.; Millington, N.J.; Pettit, G.R.; Bibby, M.C. Auristatin PYE, a novel synthetic derivative of dolastatin 10, is highly effective in human colon tumour models. Int. J. Oncol. 2007, 31, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Prokopiou, E.M.; Cooper, P.A.; Pettit, G.R.; Bibby, M.C.; Shnyder, S.D. Potentiation of the activity of cisplatin in a human colon tumour xenograft model by auristatin PYE, a structural modification of dolastatin 10. Mol. Med. Rep. 2010, 3, 309–313. [Google Scholar] [PubMed]
- Piplani, H.; Rana, C.; Vaish, V.; Vaiphei, K.; Sanyal, S.N. Dolastatin, along with Celecoxib, stimulates apoptosis by a mechanism involving oxidative stress, membrane potential change and PI3-K/AKT pathway down regulation. Biochim. Biophys. Acta 2013, 1830, 5142–5156. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Engene, N.; Paul, V.J.; Luesch, H. Targeted natural products discovery from marine cyanobacteria using combined phylogenetic and mass spectrometric evaluation. J. Nat. Prod. 2015, 78, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.H.; Chai, C.L.; Heath, G.A.; Mahon, P.J.; Smith, G.D.; Waring, P.; Wilkes, B.A. Synthesis, electrochemistry, and bioactivity of the cyanobacterial calothrixins and related quinones. J. Med. Chem. 2004, 47, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Santarsiero, B.D.; Kim, H.; Krunic, A.; Shen, Q.; Swanson, S.M.; Chai, H.; Kinghorn, A.D.; Orjala, J. Merocyclophanes A and B, antiproliferative cyclophanes from the cultured terrestrial Cyanobacterium Nostoc sp. Phytochemistry 2012, 79, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Leaõ, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of Hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS ONE 2013, 8, e69562. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; Jacques, C. Epidermal barrier function and systemic diseases. Clin. Dermatol. 2012, 30, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2014, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Skin cancer: Role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health 2014, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Halliday, G.M.; Damian, D.L. Non-melanoma skin cancer: Carcinogenesis and chemoprevention. Pathology 2013, 45, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.; Basset-Seguin, N.; Dummer, R.; Lewis, K.; Schadendorf, D.; Sekulic, A.; Hou, J.; Wang, L.; Yue, H.; Hauschild, A. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. Eur. J. Cancer 2014, 50, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Berwick, M.; Pestak, C.; Thomas, N. Solar ultraviolet exposure and mortality from skin tumors. Adv. Exp. Med. Biol. 2014, 810, 342–358. [Google Scholar] [PubMed]
- Wells, J.W. Do actinic keratoses and superficial squamous cell carcinomas have a specific immunoprofile? Curr. Probl. Dermatol. 2015, 46, 36–41. [Google Scholar] [PubMed]
- Goldenberg, G.; Perl, M. Actinic keratosis: Update on field therapy. J. Clin. Aesthet. Dermatol. 2014, 7, 28–31. [Google Scholar] [PubMed]
- Kaskel, P.; Lange, U.; Sander, S.; Huber, M.A.; Utikal, J.; Leiter, U.; Krahn, G.; Meurer, M.; Kron, M. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The natural history of actinic keratosis: A systematic review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Wheller, L.; Soyer, H.P. Clinical features of actinic keratoses and early squamous cell carcinoma. Curr. Probl. Dermatol. 2015, 46, 58–63. [Google Scholar] [PubMed]
- Chetty, P.; Choi, F.; Mitchell, T. Primary care review of actinic keratosis and its therapeutic options: A global perspective. Dermatol. Ther. 2015, 5, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Suman, S.; Shukla, Y. New enlightenment of skin cancer chemoprevention through phytochemicals: In vitro and in vivo studies and the underlying mechanisms. Biomed. Res. Int. 2014, 2014, 243452. [Google Scholar] [CrossRef] [PubMed]
- Nindl, I.; Gottschling, M.; Krawtchenko, N.; Lehmann, M.D.; Rowert-Huber, J.; Eberle, J.; Stockfleth, E.; Forschner, T. Low prevalence of p53, p16(INK4a) and Ha-ras tumour-specific mutations in low-graded actinic keratosis. Br. J. Dermatol. 2007, 156, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M. Oncogenes in melanoma: An update. Eur. J. Cell. Biol. 2014, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hensler, S.; Mueller, M.M. Inflammation and skin cancer: Old pals telling new stories. Cancer J. 2013, 19, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Perez-Plasencia, C.; Alvarez-Sanchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, J.E.; Kang, O.H.; Mun, S.H.; Seo, Y.S.; Kang, D.H.; Yang, D.W.; Ryu, S.Y.; Lee, Y.M.; Kwon, D.Y. Protective effect of ixerisoside A against UVB-induced pro-inflammatory cytokine production in human keratinocytes. Int. J. Mol. Med. 2015, 35, 1411–1418. [Google Scholar] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-kappaB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, C.; Tao, Y.; Wang, S.; Wei, Z.; Cao, Y.; Wu, H.; Fan, F.; Lin, C.; Shan, Y.; et al. Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice. Food Chem. Toxicol. 2015, 82, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.G.; Blunt, M.D.; Carter, E.; Ward, S.G. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol. Rev. 2012, 64, 1027–1054. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.J.; Lehmann, P.Z.; Li, W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFkappaB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorn, M.; Yang, J.R.; Tsai, M.L.; Lai, C.S.; Ho, C.T.; Pan, M.H. Inhibitory effects of Momordica grosvenori Swingle extracts on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mouse skin. Food Funct. 2014, 5, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, F.; Zhang, G.L. Natural products and their derivatives regulating the janus kinase/signal transducer and activator of transcription pathway. J. Asian Nat. Prod. Res. 2014, 16, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Langenbach, R. The prostaglandin E2 receptor, EP2, regulates survivin expression via an EGFR/STAT3 pathway in UVB-exposed mouse skin. Mol. Carcinog. 2011, 50, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Tsukimoto, M.; Harada, H.; Kojima, S. Involvement of P2Y6 receptor in p38 MAPK-mediated COX-2 expression in response to UVB irradiation of human keratinocytes. Radiat. Res. 2011, 175, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ivan, A.L.; Campanini, M.Z.; Martinez, R.M.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.T.; Vilela, F.M.; Martins, F.S.; Zarpelon, A.C.; Cunha, T.M.; et al. Pyrrolidine dithiocarbamate inhibits UVB-induced skin inflammation and oxidative stress in hairless mice and exhibits antioxidant activity in vitro. J. Photochem. Photobiol. B 2014, 138, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, J.; Lee, H.K.; Park, H.J.; Nam, J.; Park, G.B.; Kim, Y.S.; Cho, D.; Hur, D.Y. Selenium inhibits migration of murine melanoma cells via down-modulation of IL-18 expression. Int. Immunopharmacol. 2011, 11, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Yamada, N.; Ohyama, H.; Yamanegi, K.; Nakasho, K.; Hata, M.; Nakamura, Y.; Fukunaga, S.; Futani, H.; Yoshiya, S.; et al. Enhanced suppression of pulmonary metastasis of malignant melanoma cells by combined administration of alpha-galactosylceramide and interleukin-18. Cancer Sci. 2008, 99, 113–120. [Google Scholar] [PubMed]
- Drexler, S.K.; Bonsignore, L.; Masin, M.; Tardivel, A.; Jackstadt, R.; Hermeking, H.; Schneider, P.; Gross, O.; Tschopp, J.; Yazdi, A.S. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 18384–18389. [Google Scholar] [CrossRef] [PubMed]
- Gasparoto, T.H.; de Oliveira, C.E.; de Freitas, L.T.; Pinheiro, C.R.; Hori, J.I.; Garlet, G.P.; Cavassani, K.A.; Schillaci, R.; da Silva, J.S.; Zamboni, D.S.; et al. Inflammasome activation is critical to the protective immune response during chemically induced squamous cell carcinoma. PLoS ONE 2014, 9, e107170. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zuber, J.; Li, J. Targeting autophagy in skin diseases. J. Mol. Med. 2015, 93, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Chung, B.Y.; Kim, S.W.; Kim, C.D.; Yun, W.J.; Lee, M.W.; Choi, J.H.; Chang, S.E. Activation of autophagic pathways is related to growth inhibition and senescence in cutaneous squamous cell carcinoma. Exp. Dermatol. 2014, 23, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, N.; Takagi, A.; Ueno, T.; Ikeda, S. Inverse correlation between microtubule-associated protein 1A/1B-light chain 3 and p62/sequestosome-1 expression in the progression of cutaneous squamous cell carcinoma. J. Dermatol. 2014, 41, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.J.; McKee, C.; Birch-Machin, M.A.; Ellis, R.; Armstrong, J.L.; Lovat, P.E. Increasing the therapeutic efficacy of docetaxel for cutaneous squamous cell carcinoma through the combined inhibition of phosphatidylinositol 3-kinase/AKT signalling and autophagy. Clin. Exp. Dermatol. 2013, 38, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Serravallo, M.; Jagdeo, J.; Glick, S.A.; Siegel, D.M.; Brody, N.I. Sirtuins in dermatology: Applications for future research and therapeutics. Arch. Dermatol. Res. 2013, 305, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Soltani, K.; Shea, C.R.; Li, X.; He, Y.Y. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene 2015, 34, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Qiang, L.; Zhao, B.; He, Y.Y. Mammalian SIRT2 inhibits keratin 19 expression and is a tumor suppressor in skin. Exp. Dermatol. 2014, 23, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Hida, Y.; Kubo, Y.; Murao, K.; Arase, S. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch. Dermatol. Res. 2007, 299, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Schnell, S.A.; Jacobson, E.L. Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS ONE 2012, 7, e42276. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef] [PubMed]
- Trekli, M.C.; Riss, G.; Goralczyk, R.; Tyrrell, R.M. Beta-carotene suppresses UVA-induced HO-1 gene expression in cultured FEK4. Free Radic. Biol. Med. 2003, 34, 456–464. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Kemény, Á.; Barthó, L.; Molnár, P.; Deli, J.; Szente, L.; Bozó, T.; Pál, S.; Sándor, K.; Szőke, É.; et al. Effects of some natural carotenoids on TRPA1- and TRPV1-induced neurogenic inflammatory processes in vivo in the mouse skin. J. Mol. Neurosci. 2015, 56, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jiang, S.; Levine, N.; Watson, R.R. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc. Soc. Exp. Biol. Med. 2000, 223, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Lee, S.; Lee, M.-J.; Chung, J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Kune, G.A.; Bannerman, S.; Field, B.; Watson, L.F.; Cleland, H.; Merenstein, D.; Viteta, L. Diet, alcohol, smoking, serum β-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr. Cancer 1992, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Nakajima, H.; Ohtsuki, M.; Imokawa, G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J. Dermatol. Sci. 2010, 58, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective Inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [PubMed]
- Pongcharoen, S.; Warnnissorn, P.; Lertkajornsin, O.; Limpeanchob, N. Protective effect of silk lutein on ultraviolet B-irradiated human keratinocytes. Biol. Res. 2013, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Astner, S.; Wu, A.; Chen, J.; Philips, N.; Rius-Diaz, F.; Parrado, C.; Mihm, M.; Goukassian, D.; Pathak, M.A.; González, S. Dietary lutein/zeaxanthin partially reduces photoaging and photocarcinogenesis in chronically UVB-irradiated Skh-1 hairless mice. Skin Pharmacol. Physiol. 2007, 20, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E. B.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-L.; Chiang, Y.-C.; Huang, C.-C.; Fang, J.-Y.; Chen, D.-F.; Hung, C.-F. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp. Dermatol. 2010, 19, 173–181. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Astner, S.; An, W.; Goukassian, D.; Pathak, M.A. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Article, O.; Zheng, J.; Piao, M.J.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Fucoxanthin protects cultured human keratinocytes against oxidative stress by blocking free radicals and inhibiting apoptosis. Biomol. Ther. 2013, 21, 270–276. [Google Scholar]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.-J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Thematic review series: Skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid. Res. 2007, 48, 2531–2546. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Massey, K.; Nicolaou, A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011, 19, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.C.; de Mello-Sampayo, C.; Antoniazzi, C.T.; Segat, H.J.; Silva, H.; Veit, J.C.; Piccolo, J.; Emanuelli, T.; Bürger, M.E.; Silva-Lima, B.; et al. Oral supplementation with fish oil reduces dryness and pruritus in the acetone-induced dry skin rat model. J Dermatol Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid. Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Storey, A.; McArdle, F.; Friedmann, P.S.; Jackson, M.J.; Rhodes, L.E. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-alpha-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J. Investig. Dermatol. 2005, 124, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Cansell, M.S.; Moussaoui, N.; Mancini, M. Prostaglandin E2 and interleukin-8 production in human epidermal keratinocytes exposed to marine lipid-based liposomes. Int. J. Pharm. 2007, 343, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Chene, G.; Dubourdeau, M.; Balard, P.; Escoubet-Lozach, L.; Orfila, C.; Berry, A.; Bernad, J.; Aries, M.F.; Charveron, M.; Pipy, B. n-3 and n-6 polyunsaturated fatty acids induce the expression of COX-2 via PPARgamma activation in human keratinocyte HaCaT cells. Biochim. Biophys. Acta 2007, 1771, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Nagakura, T.; Masaki, T.; Maekawa, K.; Yamashita, K. Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclo-oxygenase-2 in cultured human mast cells. Clin. Exp. Allergy 1999, 29, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Vanderveen, E.E.; Grekin, R.C.; Swanson, N.A.; Kragballe, K. Arachidonic acid metabolites in cutaneous carcinomas. Evidence suggesting that elevated levels of prostaglandins in basal cell carcinomas are associated with an aggressive growth pattern. Arch. Dermatol. 1986, 122, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Black, H.S. Modification of membrane composition, eicosanoid metabolism, and immunoresponsiveness by dietary omega-3 and omega-6 fatty acid sources, modulators of ultraviolet-carcinogenesis. Photochem. Photobiol. 1991, 54, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Shin, C.M.; Park, C.H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J Lipid. Res. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.K.; Kang, M.K.; Lee, G.T.; Lee, K.K.; Lee, H.S.; Woo, W.H.; Mun, Y.J. EPA attenuates ultraviolet radiation-induced downregulation of aquaporin-3 in human keratinocytes. Arch. Pharm. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.J.; Kim, E.J.; Oh, I.K.; Kim, Y.K.; Park, C.H.; Chung, J.H. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J. Korean Med. Sci. 2010, 25, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Campelo, A.P.; Campelo, M.W.; Brito, G.A.; Jamacaru, F.V.; Leitao, R.F.; Vasconcelos, P.R. Oil mixes omega 9, 6 and 3, enriched with seaweed, promoted reduction of thermal burned modulating NF-kB and Ki-67. Acta Cir. Bras. 2015, 30, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Thornby, J.I.; Gerguis, J.; Lenger, W. Influence of dietary omega-6, -3 fatty acid sources on the initiation and promotion stages of photocarcinogenesis. Photochem Photobiol. 1992, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Peng, Q.Y.; Li, T.; Medvecky, C.M.; Lin, Y.; Shih, W.J.; Conney, A.H.; Shapses, S.; Wagner, G.C.; Lu, Y.P. Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis 2011, 32, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Shahbakhti, H.; Watson, R.E.; Azurdia, R.M.; Ferreira, C.Z.; Garmyn, M.; Rhodes, L.E. Influence of eicosapentaenoic acid, an omega-3 fatty acid, on ultraviolet-B generation of prostaglandin-E2 and proinflammatory cytokines interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-8 in human skin in vivo. Photochem. Photobiol. 2004, 80, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Shahbakhti, H.; Azurdia, R.M.; Moison, R.M.; Steenwinkel, M.J.; Homburg, M.I.; Dean, M.P.; McArdle, F.; van Henegouwen, G.M.J.B.; Epe, B.; et al. Effect of eicosapentaenoic acid, an omega-3 polyunsaturated fatty acid, on UVR-related cancer risk in humans. An assessment of early genotoxic markers. Carcinogenesis 2003, 24, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Massey, K.A.; Bennett, S.P.; Al-Aasswad, N.M.; Roshdy, K.; Gibbs, N.K.; Friedmann, P.S.; Nicolaou, A.; Rhodes, L.E. Randomized controlled trial of oral omega-3 PUFA in solar-simulated radiation-induced suppression of human cutaneous immune responses. Am. J. Clin. Nutr. 2013, 97, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Rhodes, L.E.; Al-Aasswad, N.M.; Massey, K.A.; Nicolaou, A. Impact of EPA ingestion on COX- and LOX-mediated eicosanoid synthesis in skin with and without a pro-inflammatory UVR challenge—Report of a randomised controlled study in humans. Mol Nutr. Food. Res. 2014, 58, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, S.C.; Hughes, M.C.; Green, A.C.; van der Pols, J.C. Plasma omega-3 and omega-6 concentrations and risk of cutaneous basal and squamous cell carcinomas in Australian adults. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Huang, Z.; Giovannucci, E.; Rimm, E.B.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am. J. Clin. Nutr. 2000, 71, 135–141. [Google Scholar] [PubMed]
- Matsumoto, Y.; Sahara, H.; Fujita, T.; Hanashima, S.; Yamazaki, T.; Takahashi, S.; Sugawara, F.; Mizushina, Y.; Ohta, K.; Takahashi, N.; et al. A novel immunosuppressive agent, SQDG, derived from sea urchin. Transplant. Proc. 2000, 32, 2051–2053. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Tringali, C.; Franchini, L.; Cirillo, F.; Venerando, B. Glycoglycerolipid analogues inhibit PKC translocation to the plasma membrane and downstream signaling pathways in PMA-treated fibroblasts and human glioblastoma cells, U87MG. Eur. J. Med. Chem. 2011, 46, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Toma, L.; Kuchide, M.; Tokuda, H.; Nishino, H. Anti-tumor-promoting effects of glycoglycerolipid analogues on two-stage mouse skin carcinogenesis. Cancer Lett. 2000, 161, 201–205. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Carvedilol and spirulina may provide important health protection to smokers and other nicotine addicts: A call for pertinent research. Mol. Med. 2015, 112, 72–75. [Google Scholar]
- Yang, F.; Wong, K.H.; Yang, Y.; Li, X.; Jiang, J.; Zheng, W.; Wu, H.; Chen, T. Purification and in vitro antioxidant activities of tellurium-containing phycobiliproteins from tellurium-enriched Spirulina platensis. Drug. Des. Devel. Ther. 2014, 8, 1789–1800. [Google Scholar] [PubMed]
- Gupta, N.K.; Gupta, K.P. Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol-13-acetate. Environ. Toxicol. Pharmacol. 2012, 34, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Singh, S.P.; Hader, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B 2007, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Lee, H.W.; Jung, J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Yakovleva, I.; Bhagooli, R.; Takemura, A.; Hidaka, M. Differential susceptibility to oxidative stress of two scleractinian corals: Antioxidant functioning of mycosporine-glycine. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Wu, Q. Protective effects of mycosporine-like amino acids of Synechocystis sp. PCC 6803 and their partial characterization. J. Photochem. Photobiol. B 2007, 86, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; You, D.H.; Han, T.; Choi, E.M. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B 2014, 141, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.P., Jr.; Loh, S.P.; Fatimah, M.Y.; Perumal, K. Bioaccessibility of Carotenoids and Tocopherols in Marine Microalgae, Nannochloropsis sp. and Chaetoceros sp. Malays J. Nutr. 2009, 15, 77–86. [Google Scholar] [PubMed]
- Carballo-Cardenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (alpha-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef]
- Pal, A.; Alam, S.; Singhal, J.; Kumar, R.; Ansari, K.M.; Das, M. Protective effect of topical application of alpha-tocopherol and/or N-acetyl cysteine on argemone oil/alkaloid-induced skin tumorigenesis in mice. Nutr. Cancer 2013, 65, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ames, B.N. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003, 17, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lykkesfeldt, J.; Shigenaga, M.K.; Shigeno, E.T.; Christen, S.; Ames, B.N. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol. Med. 2002, 33, 1534–1542. [Google Scholar] [CrossRef]
- Yoshida, E.; Watanabe, T.; Takata, J.; Yamazaki, A.; Karube, Y.; Kobayashi, S. Topical application of a novel, hydrophilic gamma-tocopherol derivative reduces photo-inflammation in mice skin. J. Investig. Dermatol. 2006, 126, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Trevithick, J.R.; Xiong, H.; Lee, S.; Shum, D.T.; Sanford, S.E.; Karlik, S.J.; Norley, C.; Dilworth, G.R. Topical tocopherol acetate reduces post-UVB, sunburn-associated erythema, edema, and skin sensitivity in hairless mice. Arch. Biochem. Biophys. 1992, 296, 575–582. [Google Scholar] [CrossRef]
- Rahman, S.; Bhatia, K.; Khan, A.Q.; Kaur, M.; Ahmad, F.; Rashid, H.; Athar, M.; Islam, F.; Raisuddin, S. Topically applied vitamin E prevents massive cutaneous inflammatory and oxidative stress responses induced by double application of 12-O-tetradecanoylphorbol-13-acetate (TPA) in mice. Chem. Biol. Interact. 2008, 172, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Magdaleno, M. Topical vitamin E inhibition of immunosuppression and tumorigenesis induced by ultraviolet irradiation. Nutr. Cancer 1991, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Miller, K. The ascorbic acid content of eleven species of microalgae used in mariculture. J. Appl. Phycol. 1992, 4, 205–215. [Google Scholar] [CrossRef]
- Maia Campos, P.M.; Gianeti, M.D.; Camargo, F.B., Jr.; Gaspar, L.R. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Gehin, A.; Guyon, C.; Nicod, L. Glyphosate-induced antioxidant imbalance in HaCaT: The protective effect of Vitamins C and E. Environ. Toxicol. Pharmacol. 2006, 22, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Qin, H.H.; Wu, W.Y.; He, S.J.; Xu, J.H. Vitamin C protects against UV irradiation-induced apoptosis through reactivating silenced tumor suppressor genes p21 and p16 in a Tet-dependent DNA demethylation manner in human skin cancer cells. Cancer Biother. Radiopharm. 2014, 29, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Kim, S.I.; Bates, S.E.; Pfeifer, G.P. Riboflavin activated by ultraviolet A1 irradiation induces oxidative DNA damage-mediated mutations inhibited by vitamin C. Proc. Natl. Acad. Sci. USA 2007, 104, 5953–5958. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Ardekani, M.R.; Nabavi, S.M.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn. Mag. 2012, 8, 60–64. [Google Scholar] [PubMed]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152-6209. https://doi.org/10.3390/md13106152
Talero E, García-Mauriño S, Ávila-Román J, Rodríguez-Luna A, Alcaide A, Motilva V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Marine Drugs. 2015; 13(10):6152-6209. https://doi.org/10.3390/md13106152
Chicago/Turabian StyleTalero, Elena, Sofía García-Mauriño, Javier Ávila-Román, Azahara Rodríguez-Luna, Antonio Alcaide, and Virginia Motilva. 2015. "Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer" Marine Drugs 13, no. 10: 6152-6209. https://doi.org/10.3390/md13106152