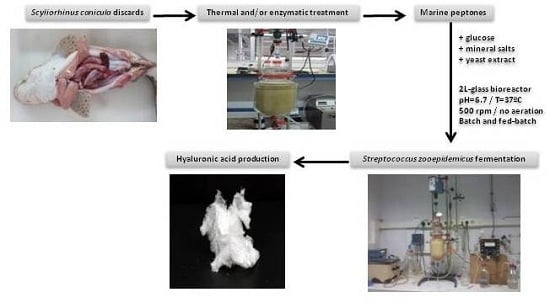
Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards
Abstract
:1. Introduction
2. Results and Discussion

| Parameters | Medium A | Medium B | Medium C | CM |
|---|---|---|---|---|
| Xm (g/L) | 3.43 ± 0.15 | 3.80 ± 0.20 | 4.61 ± 0.26 | 4.05 ± 0.16 |
| vx (g·L−1·h−1) | 0.461 ± 0.009 | 0.587 ± 0.131 | 0.835 ± 0.230 | 1.11 ± 0.33 |
| λx (h) | 2.03 ± 0.77 | 3.92 ± 0.81 | 4.15 ± 0.86 | 3.99 ± 0.62 |
| τx (h) | 5.75 ± 0.44 | 7.15 ± 0.47 | 6.91 ± 0.48 | 5.80 ± 0.28 |
| μx (h−1) | 0.538 ± 0.109 | 0.618 ± 0.151 | 0.725 ± 0.488 | 1.10 ± 0.32 |
| tx (h) | 9.46 ± 0.80 | 10.39 ± 0.67 | 9.66 ± 0.71 | 7.62 ± 0.34 |
| YX/G | 0.058 | 0.071 | 0.075 | 0.080 |
| YX/Pr | 1.632 | 1.254 | 0.991 | 1.275 |
| R2 | 0.994 | 0.994 | 0.993 | 0.995 |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 |
| Hm (g/L) | 2.26 ± 0.33 | 1.51 ± 0.06 | 2.32 ± 0.13 | 3.03 ± 0.14 |
| vh (g·L−1·h−1) | 0.207 ± 0.053 | 0.225 ± 0.028 | 0.456 ± 0.126 | 0.667 ± 0.169 |
| λh (h) | 4.23 ± 1.46 | 6.79 ± 0.45 | 5.14 ± 0.79 | 4.81 ± 0.66 |
| τh (h) | 9.69 ± 1.33 | 10.15 ± 0.29 | 7.68 ± 0.44 | 7.09 ± 0.36 |
| μh (h−1) | 0.366 ± 0.128 | 0.596 ± 0.085 | 0.785 ± 0.232 | 0.879 ± 0.235 |
| th (h) | 15.15 ± 2.01 | 13.50 ± 0.56 | 10.23 ± 0.89 | 9.36 ± 0.40 |
| YH/G | 0.040 | 0.029 | 0.044 | 0.061 |
| YH/Pr | 1.143 | 0.512 | 0.584 | 0.967 |
| YH/X | 0.700 | 0.408 | 0.589 | 0.759 |
| R2 | 0.986 | 0.998 | 0.993 | 0.995 |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 |
| Lm (g/L) | 35.78 ± 2.28 | 34.60 ± 1.14 | 40.74 ± 1.82 | 30.25 ± 0.77 |
| vl (g·L−1·h−1) | 3.46 ± 0.54 | 3.90 ± 0.29 | 5.73 ± 0.89 | 7.81 ± 1.39 |
| λl (h) | 4.31 ± 0.84 | 5.61 ± 0.35 | 5.09 ± 0.61 | 4.34 ± 0.38 |
| τl (h) | 9.48 ± 0.71 | 10.05 ± 0.27 | 8.65 ± 0.38 | 6.28 ± 0.20 |
| μl (h−1) | 0.387 ± 0.079 | 0.451 ± 0.042 | 0.562 ± 0.099 | 1.03 ± 0.19 |
| tl (h) | 14.65 ± 1.34 | 14.48 ± 0.59 | 12.21 ± 0.70 | 8.21 ± 0.25 |
| YL/G | 0.651 | 0.643 | 0.713 | 0.622 |
| YL/Pr | 18.44 | 11.29 | 9.42 | 9.87 |
| R2 | 0.995 | 0.999 | 0.997 | 0.998 |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 |

| Parameters | Medium A | Medium C | CM |
|---|---|---|---|
| Xm (g/L) | 3.34 ± 0.16 | 3.27 ± 0.17 | 4.56 ± 0.29 |
| vx (g·L−1·h−1) | 0.467 ± 0.098 | 0.464 ± 0.103 | 0.930 ± 0.350 |
| λx (h) | 2.47 ± 0.83 | 2.69 ± 0.87 | 3.46 ± 1.03 |
| τx (h) | 6.05 ± 0.48 | 6.22 ± 0.50 | 8.34 ± 0.77 |
| μx (h−1) | 0.559 ± 0.128 | 0.567 ± 0.138 | 0.820 ± 0.321 |
| tx (h) | 9.63 ± 0.60 | 9.74 ± 0.87 | 7.62 ± 0.34 |
| YX/G | 0.047 | 0.049 | 0.052 |
| YX/Pr | 1.201 | 0.976 | 1.027 |
| R2 | 0.994 | 0.994 | 0.988 |
| p-Value | <0.001 | <0.001 | <0.001 |
| Hm (g/L) | 2.53 ± 0.09 | 2.66 ± 0.17 | 3.23 ± 0.25 |
| vh (g L−1·h−1) | 0.332 ± 0.035 | 0.321 ± 0.054 | 0.589 ± 0.234 |
| λh (h) | 5.19 ± 0.44 | 5.42 ± 0.75 | 3.76 ± 1.22 |
| τh (h) | 9.00 ± 0.27 | 9.56 ± 0.52 | 6.50 ± 0.65 |
| μh (h−1) | 0.526 ± 0.061 | 0.483 ± 0.095 | 0.730 ± 0.299 |
| th (h) | 12.80 ± 0.99 | 13.70 ± 0.61 | 9.24 ± 0.81 |
| YH/G | 0.035 | 0.039 | 0.039 |
| YH/Pr | 0.896 | 0.772 | 0.767 |
| YH/X | 0.746 | 0.791 | 0.747 |
| R2 | 0.999 | 0.996 | 0.986 |
| p-Value | <0.001 | <0.001 | <0.001 |
| Lm (g/L) | 50.50 ± 5.44 | 51.13 ± 4.16 | 81.88 ± 19.65 |
| vl (g·L−1·h−1) | 4.68 ± 0.83 | 5.08 ± 0.76 | 6.53 ± 1.92 |
| λl (h) | 5.01 ± 0.99 | 5.11 ± 0.78 | 4.49 ± 1.89 |
| τl (h) | 10.42 ± 0.94 | 10.14 ± 0.69 | 10.76 ± 2.22 |
| μl (h−1) | 0.370 ± 0.090 | 0.398 ± 0.079 | 0.319 ± 0.143 |
| tl (h) | 15.82 ± 2.79 | 15.16 ± 2.19 | 17.03 ± 4.01 |
| YL/G | 0.700 | 0.762 | 0.895 |
| YL/Pr | 17.99 | 15.04 | 17.77 |
| R2 | 0.994 | 0.996 | 0.978 |
| p-Value | <0.001 | <0.001 | <0.001 |

3. Experimental Section
3.1. Preparation of Protein Substrate (Peptones)
3.2. Bacterial Strain
3.3. Microbiological Culture Media and Growth Conditions
3.4. Analytical Methods
3.5. Modeling of S. zooepidemicus Cultivation
3.6. Numerical and Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shiedlin, A.; Bigelow, R.; Christopher, W.; Arbabi, S.; Yang, L.; Maier, R.V.; Wainwright, N.; Childs, A.; Miller, R.J. Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 2004, 5, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kawasaki, T. Microbial synthesis of hyaluronan and chitin: New approaches. J. Biosci. Bioeng. 2005, 99, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohyd. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Soltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.P.; Murado, M.A. Chondroitin sulphate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid solutions—A processing method for efficient chemical modification. J. Appl. Polym. Sci. 2013, 130, 145–152. [Google Scholar] [CrossRef]
- Amagai, I.; Tashiro, Y.; Ogawa, H. Improvement of the extraction procedure for hyaluronan from fish eyeball and the molecular characterization. Fish. Sci. 2009, 75, 805–810. [Google Scholar] [CrossRef]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Proc. 2012, 90, 491–498. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.Y.; Kin, C.W. A novel approach to the production of hyaluronic acid by Streptococcus zooepidemicus. J. Microbiol. Biotechnol. 2006, 16, 1849–1855. [Google Scholar]
- Cooney, M.J.; Goh, L.T.; Lee, P.L.; Johns, M.R. Structured model based analysis and control of the hyaluronic acid fermentation by Streptococcus zooepidemicus: Physiological implications of glucose and complex nitrogen-limited growth. Biotechnol. Prog. 1999, 15, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. High production of hyaluronic and lactic acids by Streptococcus zooepidemicus in fed-batch cultures using commercial and marine peptones from fishing by-products. Biochem. Eng. J. 2009, 44, 125–130. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Pérez, N. Importance of discarded blue whiting (Micromesistius poutassou) in the diet of the lesser spotted dogfish (Scyliorhinus canicula) in the Cantabrian Sea. ICES J. Mar. Sci. 1998, 55, 331–341. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Fernández, A.; Olaso, I.; Sánchez, F.; Gancedo, R.; Punzón, A.; Cendrero, O. Overview of continental shelf elasmobranch fisheries in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 375–385. [Google Scholar] [CrossRef]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Factories 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.R.; Goh, L.T.; Oeggerli, A. Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus. Biotechnol. Lett. 1994, 16, 507–512. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Johns, M.R. Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl. Environ. Microbiol. 1997, 63, 2759–2764. [Google Scholar] [PubMed]
- De Macedo, A.C.; Santana, M. Hyaluronic acid depolymerization by ascorbate-redox effects on solid state cultivation of Streptococcus zooepidemicus in cashew apple fruit bagasse. World J. Microbiol. Biotechnol. 2012, 28, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Macedo, A.; Eguchi, S.; Santana, M. Microbial production of hyaluronic acid from agricultural resource derivatives. Bioresour. Technol. 2010, 101, 6506–6509. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.C.; Vignoli, J.A.; Baldo, C.; Pereira, H.C.B.; da Silva, R.S.S.F.; Celligoi, M.A.P.C. Agroindustrial byproducts for the production of hyaluronic acid by Streptococcus zooepidemicus ATCC 39920. Int. J. Sci. Technol. Res. 2015, 4, 114–118. [Google Scholar]
- Fong Chong, B.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Enhanced hyaluronic acid production by a two-stage culture strategy based on the modeling of batch and fed-batch cultivation of Streptococcus zooepidemicus. Biores. Technol. 2008, 99, 8532–8536. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, S.J.; Chen, T.L. Production of hyaluronic acid by repeated batch fermentation. Biochem. Eng. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Guerra, N.P.; Agrasar, A.T.; Macías, C.L.; Pastrana, L. Modelling the fed-batch production of pediocin using mussel processing wastes. Proc. Biochem. 2005, 40, 1071–1083. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Mirón, J.; González, M.P.; Murado, M.A. Bacteriocin production and pH gradient. Some mathematical models and their problems. Enzyme Microb. Technol. 2005, 37, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Guerra, N.P.; Bernárdez, P.F.; Pastrana, L. Fed-batch pediocin production on whey using different feeding media. Enzyme Microb. Technol. 2007, 41, 397–406. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 270, 27299–27304. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Murado, M.A.; Vázquez, J.A.; Montemayor, M.I.; Cabo, M.L.; González, M.P. Two mathematical models for the correction of carbohydrate and protein interference in the determination of uronic acids by the m-hydroxydiphenyl method. Biotechnol. Appl. Biochem. 2005, 41, 209–216. [Google Scholar] [PubMed]
- Bernfeld, P. Enzymes of starch degradation and synthesis. Adv. Enzymol. 1951, 12, 379–427. [Google Scholar]
- Vázquez, J.A.; Lorenzo, J.M.; Fuciños, P.; Franco, D. Evaluation of non-linear equations to model different animal growths with mono and bi-sigmoid profiles. J. Theor. Biol. 2012, 314, 95–105. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards. Mar. Drugs 2015, 13, 6537-6549. https://doi.org/10.3390/md13106537
Vázquez JA, Pastrana L, Piñeiro C, Teixeira JA, Pérez-Martín RI, Amado IR. Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards. Marine Drugs. 2015; 13(10):6537-6549. https://doi.org/10.3390/md13106537
Chicago/Turabian StyleVázquez, José A., Lorenzo Pastrana, Carmen Piñeiro, José A. Teixeira, Ricardo I. Pérez-Martín, and Isabel R. Amado. 2015. "Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards" Marine Drugs 13, no. 10: 6537-6549. https://doi.org/10.3390/md13106537
APA StyleVázquez, J. A., Pastrana, L., Piñeiro, C., Teixeira, J. A., Pérez-Martín, R. I., & Amado, I. R. (2015). Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards. Marine Drugs, 13(10), 6537-6549. https://doi.org/10.3390/md13106537