2.2. LMWF and Level of Glycosaminoglycan Chains Expressed in HUVECs
We first investigated whether LMWF could modify the GAG chain level expressed by HUVECs. For that purpose, the level of total GAGs, HS chains, and chondroitin sulfate (CS) chains were determined by DMMB assays in the lysate of endothelial cells after 24 h of 10 μg/mL LMWF treatment and compared to UT control cells. There was no significant difference in the level of GAGs, HS, and CS after LMWF incubation (
Figure S1). Following LMWF treatment, the amount of total GAGs in the conditioned medium of LMWF-treated cells decreased by 28% ± 8% at 24 h, as compared to untreated control cells (
p < 0.05,
n = 3,
Figure 2A). Further analysis revealed that HS amounts decreased by 25% ± 5% in the conditioned medium of the LMWF-treated cells, whereas there was no variation of CS chain amount (
Figure 2A). These data suggests that LMWF may modify the HS and HSPG turnover (HS synthesis or cleavage and HSPG shedding).
Figure 1.
Effects of Low molecular weight fucoidan (LMWF) on endothelial cell abilities: migration and 2D-angiogenesis. Human vascular endothelial cells (HUVEC) were incubated with 10 µg/mL LMWF for 24 h and the migration (A), the lamellipodia formation (B) and the capillary tube formation (length and area) (C) were determined. (A) Migration chamber assay. HUVECs incubated with or without 10 μg/mL LMWF, were allowed to migrate through the porous fibronectin-coated membrane. They were stained with Mayer’s hemalum and counted. The results are expressed as cell number per field; (B) Lamellipodia formation. LMWF induced the formation of lamellipodia and ruffles (white arrows indicate lamellipodia/ruffle formation, DAPI-nucleus (blue), Phalloidin-F-actin (red)). Bar = 10 µm; (C) Capillary tube formation (2D-angiogenesis assay) on Matrigel. Left and right panels show the length (left) and area (right) of endothelial capillaries formed by HUVECs treated with or without 10 µg/mL LMWF. Lower right panel shows a representative image of capillary network, as photographed with phase contrast microscopy (magnification ×100). * p < 0.05 versus control untreated (UT) cells. A.U.: arbitrary unit.
Figure 1.
Effects of Low molecular weight fucoidan (LMWF) on endothelial cell abilities: migration and 2D-angiogenesis. Human vascular endothelial cells (HUVEC) were incubated with 10 µg/mL LMWF for 24 h and the migration (A), the lamellipodia formation (B) and the capillary tube formation (length and area) (C) were determined. (A) Migration chamber assay. HUVECs incubated with or without 10 μg/mL LMWF, were allowed to migrate through the porous fibronectin-coated membrane. They were stained with Mayer’s hemalum and counted. The results are expressed as cell number per field; (B) Lamellipodia formation. LMWF induced the formation of lamellipodia and ruffles (white arrows indicate lamellipodia/ruffle formation, DAPI-nucleus (blue), Phalloidin-F-actin (red)). Bar = 10 µm; (C) Capillary tube formation (2D-angiogenesis assay) on Matrigel. Left and right panels show the length (left) and area (right) of endothelial capillaries formed by HUVECs treated with or without 10 µg/mL LMWF. Lower right panel shows a representative image of capillary network, as photographed with phase contrast microscopy (magnification ×100). * p < 0.05 versus control untreated (UT) cells. A.U.: arbitrary unit.
![Marinedrugs 13 06588 g001]()
Figure 2.
LMWF and glycosaminoglycan chain level in HUVECs. (A) Glycosaminoglycan quantification. HUVECs were incubated with 10 µg/mL LMWF for 24 h and the amount of total GAGs, CS and HS chains were determined in the supernatant according to a dimethyl-methylene blue (DMMB) assay; (B) Exostosin-1 and -2 (EXT1 or EXT2) mRNA levels were determined by real-time RT-PCR in cells treated with or without 10 µg/mL LMWF. (C) EXT1 or EXT2 protein levels were determined by western blot in cells treated with or without 10 µg/mL LMWF. Right panel shows a representative image of the western blot assay. * p < 0.05 versus control untreated (UT) cells. A.U.: arbitrary unit.
Figure 2.
LMWF and glycosaminoglycan chain level in HUVECs. (A) Glycosaminoglycan quantification. HUVECs were incubated with 10 µg/mL LMWF for 24 h and the amount of total GAGs, CS and HS chains were determined in the supernatant according to a dimethyl-methylene blue (DMMB) assay; (B) Exostosin-1 and -2 (EXT1 or EXT2) mRNA levels were determined by real-time RT-PCR in cells treated with or without 10 µg/mL LMWF. (C) EXT1 or EXT2 protein levels were determined by western blot in cells treated with or without 10 µg/mL LMWF. Right panel shows a representative image of the western blot assay. * p < 0.05 versus control untreated (UT) cells. A.U.: arbitrary unit.
2.3. LMWF and Heparan Sulfate Biosynthesis and Degradation Enzymes in HUVECs
We have first studied the effects of LMWF on enzymes involved in HS biosynthesis (EXT1, EXT2) or degradation (heparanase). These glycosyltransferases EXT1 and EXT2 are responsible for the elongation of HS by catalyzing the addition of alternating β-
d-glucuronate (GlcA) and α-
d-
N-acetylglucosamine (GlcNAc) units to the tetrasaccharide linker of GAGs. We assessed the glycosaminoglycan polymerization (
EXT1 and
EXT2) mRNA levels by quantitative RT-PCR. The mRNA expression level of
EXT2 in LMWF-treated cells was decreased by 36% ± 13%, as compared to untreated cells at 24 h (
p < 0.05), whereas the level of mRNA encoding for
EXT1 was unaffected (
Figure 2B). The EXT1 and EXT2 protein levels were measured by western blot analysis in HUVEC lysates. A slightly decreased EXT2 level by 23% ± 5% was observed in the LMWF-treated cells (
p < 0.05). No significant difference was observed for EXT1 (
Figure 2C).
The HS-degrading enzyme heparanase (HPSE) is an endo-β-
d-glucuronidase, which plays an important role in remodeling of the basement membrane and extracellular matrix during process of inflammation [
13,
14,
15]. HPSE is synthesized as an inactive 65 kDa pro-form enzyme (pro-HPSE), can be transformed into active heterodimer consisting of 50 and 8 kDa subunits (active HPSE), and cleaves HS chains attached to proteoglycans, such as syndecans and perlecan [
15,
16]
. HPSE mRNA expression, as assessed by quantitative RT-PCR, was increased by 2.4 fold in LMWF-treated cells, as compared to untreated control cells (
Figure 3A). Active HPSE form (50 kDa) expression, as assessed by western blot, was significantly increased up to 2 fold in the supernatant or by 20% ± 5% in the lysate of the LMWF-treated cells (
Figure 4B). HPSE activity was slightly but significantly increased up to 20% ± 3% in the lysate of LMWF-treated cells (
Figure 3C), whereas it was not detected in the respective conditioned medium.
Figure 3.
LMWF and heparanase in HUVECs. (A) HPSE mRNA levels were determined by real-time RT-PCR in cells treated with or without 10 µg/mL LMWF; (B) HPSE protein levels were determined by western blot in the supernatant or in the lysate of cells treated with or without 10 µg/mL LMWF. Lower panel shows a representative image of the western blot assay. (C) Heparanase activity was checked in the lysate of LMWF-treated cells. HPSE activity in untreated cells was arbitrary set to 100%. * p < 0.05, *** p < 0.0005, LMWF-treated cells versus LMWF-untreated cells (UT). A.U.: arbitrary unit.
Figure 3.
LMWF and heparanase in HUVECs. (A) HPSE mRNA levels were determined by real-time RT-PCR in cells treated with or without 10 µg/mL LMWF; (B) HPSE protein levels were determined by western blot in the supernatant or in the lysate of cells treated with or without 10 µg/mL LMWF. Lower panel shows a representative image of the western blot assay. (C) Heparanase activity was checked in the lysate of LMWF-treated cells. HPSE activity in untreated cells was arbitrary set to 100%. * p < 0.05, *** p < 0.0005, LMWF-treated cells versus LMWF-untreated cells (UT). A.U.: arbitrary unit.
2.4. Effects of LMWF on the Syndecan Expression
We therefore focused on the effect of LMWF on the expression of the heparan sulfate transmembrane proteoglycans belonging to the syndecan family, SDC-1 and SDC-4.
In HUVECs, we demonstrated that LMWF increased the level of mRNA encoding for SDC-1 by 48% ± 12% (
p < 0.005), whereas it decreased that of SDC-4 by 38% ± 9% (
p < 0.05) (
Figure 4A). We then analyzed SDC-1 and SDC-4 levels by western blot. The SDCs contains the core protein (ectodomain, transmembrane, and cytoplasmic domains) and the GAG, which are attached in the ectodomain part of SDCs. As shown previously, it is well known that the western blot expression pattern is heterogeneous (many bands from 20 kDa to 250 kDa) and show many forms of SDC-1 and SDC-4 in the cell lysate [
17]. Our results of SDC protein expression showed the presence of 3 different forms for SDC-1 and SDC-4 (
Figure 4B,C). Upon LMWF treatment, protein level was significantly increased by 22% ± 2% (33 kDa), 13% ± 5% (75 kDa) and 18% ± 4% (250 kDa) for SDC-1 or decreased by 28% ± 5% (22 kDa), 41% ± 9% (75 kDa), and 48% ± 5% (150 kDa) for SDC-4, respectively (
Figure 4B,C). As assessed by dot blot, the shedded ectodomain level of SDC-1 in the supernatant of LMWF-treated cells was increased by 2 fold, as compared to untreated control cells, whereas that of SDC-4 was decreased by 35% ± 8% (
p < 0.05) (
Figure 4D).
Taken together, LMWF modulate SDC-1 and SDC-4 gene expression and ectodomain shedding in HUVEC in vitro culture.
Figure 4.
Effects of LMWF on the SDC expression in HUVECs. SDC-1 and SDC-4 mRNA or protein levels in endothelial cells treated or not with 10 µg/mL LMWF were analyzed respectively by real time RT-PCR (A) or western blot (B,C). SDC-1 and SDC-4 ectodomains in the supernatant of cells treated with or without 10 µg/mL LMWF were analyzed by dot blot (D). * p < 0.05, ** p < 0.005, significantly different to LMWF-untreated cells (UT). A.U.: arbitrary unit.
Figure 4.
Effects of LMWF on the SDC expression in HUVECs. SDC-1 and SDC-4 mRNA or protein levels in endothelial cells treated or not with 10 µg/mL LMWF were analyzed respectively by real time RT-PCR (A) or western blot (B,C). SDC-1 and SDC-4 ectodomains in the supernatant of cells treated with or without 10 µg/mL LMWF were analyzed by dot blot (D). * p < 0.05, ** p < 0.005, significantly different to LMWF-untreated cells (UT). A.U.: arbitrary unit.
In vivo, the influence of LMWF on SDCs expression was assessed in a Sprague Dawley Rat model of intimal hyperplasia. We have already used this model to show the pro-angiogenic effect of LMWF treatment [
10]. In addition, SDC-1 and SDC-4 have been shown to play an important role in the pathological response of a balloon-injured wall artery [
18]. Briefly, rats were subjected to balloon injury into the thoracic artery to create local destruction of endothelial layer leading to inflammation and intimal hyperplasia development. Two weeks after LMWF-intramuscular injection the level of SDC-1 and SDC-4 in the balloon-injured artery was analyzed.
Our results demonstrated that the expression of SDC-1 and SDC-4 was very low in healthy arteries in the media (M) and adventitia (A) layers, whereas it increased in the neointima (N), media and adventitia layers in balloon-injured, and NaCl-treated arteries (vehicle), leading to development of intimal hyperplasia (
Figure 5A–E). The LMWF treatment of injured artery increased SDC-1 expression in the neointima and in the adventitia layers, as compared to vehicle (
Figure 5C). Furthermore, upon LMWF treatment, the SDC-4 expression was decreased in the neointima and media, but largely increased in the adventitia layer, as compared to vehicle (
Figure 5F). These
in vivo results were in concordance with our previously described observation obtained in the HUVECs
in vitro culture [
10].
All these results suggest that LMWF has an important influence on the proteoglycan distribution in the endothelial cells and can increase SDC-1 and decrease SDC-4 expression in vitro and in vivo.
Figure 5.
Effects of LMWF on the SDC distribution in rat balloon injured artery. SDC-1 and SDC-4 expressions were assessed using immunohistochemistry in rat model of intimal hyperplasia. (A) SDC-1 or (D) SDC-4 expressions in non injured arteries; (B) SDC-1 or (E) SDC-4 expressions in injured arteries treated with NaCl; (C) SDC-1 or (F) SDC-4 expressions in injured arteries treated with LMWF. White arrows indicate SDC expressions in the neointima layer in high power view inserts (red). Green: autofluorescence of the elastic fibers of the lamina. Magnification ×100, L: lumen, N: neointima, M: media, A: adventitia.
Figure 5.
Effects of LMWF on the SDC distribution in rat balloon injured artery. SDC-1 and SDC-4 expressions were assessed using immunohistochemistry in rat model of intimal hyperplasia. (A) SDC-1 or (D) SDC-4 expressions in non injured arteries; (B) SDC-1 or (E) SDC-4 expressions in injured arteries treated with NaCl; (C) SDC-1 or (F) SDC-4 expressions in injured arteries treated with LMWF. White arrows indicate SDC expressions in the neointima layer in high power view inserts (red). Green: autofluorescence of the elastic fibers of the lamina. Magnification ×100, L: lumen, N: neointima, M: media, A: adventitia.
2.5. Assessment of EXT-, HPSE- or SDC Involvement in Biological Effects of LMWF
To assess the potential role of EXT1/EXT2, HPSE or SDC-1/SDC-4 in the biological effects induced by LMWF, specific siRNAs were carried out.
Quantitative RT-PCR showed that the expression of the mRNAs and proteins of EXT2 in
EXT2-siRNA- and EXT1 in
EXT1-siRNA-transfected cells was reduced up to 72% ± 16% and 71% ± 15%, respectively, as compared to the
SNC-siRNA-transfected control cells (
Figure 6A). The flow cytometry assay showed that the binding of 10E4 anti-HS antibodies to
EXT2-siRNA- or
EXT1-siRNA-transfected cells was respectively reduced by 75% ± 8% or by 80% ± 9%, as compared to
SNC-siRNA-transfected control cells (
Figure 6B).
Figure 6.
Assessment of EXT involvement in biological effects of LMWF in HUVECs. HUVECs were transfected with EXT1-siRNA or EXT2-siRNA or with SNC-siRNA control. (A) EXT1 or EXT2 mRNA levels were determined in EXT1-siRNA- or EXT2-siRNA- or SNC-siRNA-transfected control cell by real-time RT PCR. EXT1- or EXT2 mRNA level normalized to GAPDH mRNA level in SNC-siRNA-transfected control cells was arbitrarily set to 1; (B) The binding of 10E4 anti-HS antibodies to EXT2- or EXT1-siRNA transfected cells was compared to that of SNC-siRNA-transfected cells; (C) Migration was assayed in cells treated with or without 10 µg/mL LMWF; (D) 2D-angiogenesis was assayed in cells treated with or without 10 µg/mL LMWF. The difference in the capillary network length between LMWF-treated and untreated cells in each RNA interference condition (EXT1 or EXT2 silencing) was compared to that in SNC-siRNA-transfected cells. Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells. * p < 0.05, *** p < 0.0005 versus SNC-siRNA-transfected control cells. A.U.: arbitrary unit.
Figure 6.
Assessment of EXT involvement in biological effects of LMWF in HUVECs. HUVECs were transfected with EXT1-siRNA or EXT2-siRNA or with SNC-siRNA control. (A) EXT1 or EXT2 mRNA levels were determined in EXT1-siRNA- or EXT2-siRNA- or SNC-siRNA-transfected control cell by real-time RT PCR. EXT1- or EXT2 mRNA level normalized to GAPDH mRNA level in SNC-siRNA-transfected control cells was arbitrarily set to 1; (B) The binding of 10E4 anti-HS antibodies to EXT2- or EXT1-siRNA transfected cells was compared to that of SNC-siRNA-transfected cells; (C) Migration was assayed in cells treated with or without 10 µg/mL LMWF; (D) 2D-angiogenesis was assayed in cells treated with or without 10 µg/mL LMWF. The difference in the capillary network length between LMWF-treated and untreated cells in each RNA interference condition (EXT1 or EXT2 silencing) was compared to that in SNC-siRNA-transfected cells. Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells. * p < 0.05, *** p < 0.0005 versus SNC-siRNA-transfected control cells. A.U.: arbitrary unit.
![Marinedrugs 13 06588 g006]()
There were no significant difference in cell migration after LMWF treatment in
EXT1-siRNA- or
EXT2-siRNA-transfected cells, as compared to
SNC-siRNA-transfected cells (
Figure 6C). In contrast, the LMWF induction of 2D-angiogenesis was abolished in LMWF-treated
EXT2-siRNA-transfected cells by 98% ± 5%, or decreased in LMWF-treated
EXT1-siRNA
-transfected cells by 33% ± 5% (
p < 0.05), as compared to
SNC-siRNA-transfected control cells (
Figure 6D). These latter data suggest that EXT2 and, to a lesser extent EXT1, affect the pro-angiogenic effect of LMWF.
HPSE-siRNA-transfected cells were used for 2D-angiogenesis or migration assays. Quantitative RT-PCR showed that the expression of the mRNAs encoding for heparanase in
HPSE-siRNA-transfected cells was reduced up to 74% ± 8%, as compared to the
SNC-siRNA-transfected control cells (
Figure 7A). Under basal conditions (in the absence of LMWF),
HPSE-siRNA transfection decreased endothelial cell migration by 37% ± 5% (
p < 0.05), but had no effect on 2D-angiogenesis (
Figure S2A,B). However, upon LMWF stimulation and
HPSE-siRNA transfection, the ability of HUVECs to form capillary network in Matrigel 2D-angiogenesis assay was altered. The capillary network length induced by LMWF treatment was largely decreased by 51% ± 11% in
HPSE-siRNA-transfected cells, as compared to
SNC-siRNA-transfected control cells (
Figure 7B). In contrast, the cell migration induced by LMWF was significantly increased by 56% ± 9% (
p < 0.05) in
HPSE-siRNA-transfected, as compared to
SNC-siRNA-transfected cells (
Figure 7C).
Figure 7.
Assessment of HPSE involvement in biological effects of LMWF in HUVECs. HUVECs were transfected with HPSE-siRNA or SNC-siRNA control. (A) HPSE mRNA levels were determined in HPSE-siRNA- or SNC-siRNA-transfected cells by real-time RT-PCR. HPSE mRNA level normalized to GAPDH mRNA level in SNC-siRNA-transfected control cells was arbitrary set to 1; (B) 2D-angiogenesis was assayed in cells treated with or without 10 µg/mL LMWF. The difference in the capillary network length between LMWF-treated and untreated cells in HPSE RNA interference condition was compared to that in SNC-siRNA-transfected cells; (C) Migration was assayed in cells treated with or without 10 µg/mL LMWF. Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells. * p < 0.05, ** p < 0.005 versus SNC-siRNA-transfected control cells. A.U.: arbitrary unit.
Figure 7.
Assessment of HPSE involvement in biological effects of LMWF in HUVECs. HUVECs were transfected with HPSE-siRNA or SNC-siRNA control. (A) HPSE mRNA levels were determined in HPSE-siRNA- or SNC-siRNA-transfected cells by real-time RT-PCR. HPSE mRNA level normalized to GAPDH mRNA level in SNC-siRNA-transfected control cells was arbitrary set to 1; (B) 2D-angiogenesis was assayed in cells treated with or without 10 µg/mL LMWF. The difference in the capillary network length between LMWF-treated and untreated cells in HPSE RNA interference condition was compared to that in SNC-siRNA-transfected cells; (C) Migration was assayed in cells treated with or without 10 µg/mL LMWF. Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells. * p < 0.05, ** p < 0.005 versus SNC-siRNA-transfected control cells. A.U.: arbitrary unit.
LMWF biological effects have been checked in
SDC-1-siRNA- or
SDC-4-siRNA-transfected cells or in cells treated with specific anti-SDC-1 or anti-SDC-4 antibodies. As described [
19,
20], quantitative RT-PCR showed that the expression of the mRNAs encoding for SDC-1 in
SDC1-siRNA- or SDC-4 in
SDC4-siRNA-transfected cells was reduced up to 69% ± 14% and 73% ± 17% respectively, as compared to
SNC- siRNA-transfected control cells.
Under basal conditions, the 2D-angiogenenis assays showed a significant decrease in cell capillary network length by 23% ± 4% in
SDC-1- and by 54% ± 7% in
SDC-4-siRNA-transfected cells, as compared to
SNC-siRNA-transfected control cells (
Figure S3A). Upon LMWF cell treatment, the LMWF-induction of 2D-angiogenenis was unchanged in
SDC-1-siRNA-transfected cells, whereas it was significantly increased in
SDC-4-siRNA
-transfected cells by 62% ± 5% (
p < 0.005), as compared to
SNC-siRNA-transfected control cells (
Figure 8A).
Under basal conditions, endothelial cell migration assayed in modified Boyden chambers was decreased by 43% ± 5% and by 40% ± 8% in
SDC-1-siRNA-transfected and anti-SDC-1 antibody-incubated cells, respectively (
Figure S3B). LMWF induction of endothelial cell migration was decreased in
SDC-1-siRNA-transfected cells by 20% ± 5% and in anti-SDC-1 antibody-incubated cells by 47% ± 3%, as compared to respective control cells (
Figure 8B). These data were confirmed by a wound healing assay (
Figure S3C). Regarding RNA silencing experiments, these data suggest that SDC-1 does not play a crucial role in LMWF-induced effects.
Basal endothelial cell migration was decreased by 68% ± 5% and by 67% ± 9% in
SDC-4-siRNA-transfected and anti-SDC-4 antibody-incubated cells, respectively (
Figure S3B). However, LMWF-induction of endothelial cell migration was largely increased by 87% ± 5% in
SDC-4-siRNA-transfected cells or by 2 fold in anti-SDC-4-antibody incubated cells, as compared to respective control cells (
Figure 8B). These data were confirmed by a wound healing assay (
Figure S3C). These results demonstrated that SDC-4 expression limits the LMWF effect on the cells.
Altogether, we have demonstrated that on the one hand EXT2 (and EXT1 to a lesser extent) and HPSE expression, and on the other hand SDC-4, play critical roles in LMWF pro-angiogenic effects. We have then addressed the question whether silencing of endothelial HPSE or EXT2 could affect SDC-4 level. In
HPSE- or
EXT2-siRNA-transfected cells,
SDC-4 mRNA level was up-regulated respectively by 64% ± 19% or 35% ± 10%, as compared to
SNC-siRNA-transfected control cells (
Figure 8C). In addition, there was no effect on
SDC-1 mRNA level in
HPSE- or
EXT2 silenced cells (
Figure 8C).
2.6. Discussion
Fucoidan exhibits various biological effects, among them anti-inflammatory, low anti-coagulant and anti-thrombotic activities. We have previously shown the therapeutic potential of low molecular weight fucoidan (LMWF) in reduction of in-stent restenosis in a rabbit model, vascular tissue repair [
21], and in critical hind limb ischemia in a rat model [
7].
In this study, we hypothesized that LMWF could modify the amount and the distribution of heparan sulfate chains expressed in endothelial cells and of syndecan-1 (SDC-1) and syndecan-4 (SDC-4), two major heparan sulfate (HS) membrane proteoglycans.
Figure 8.
Assessment of SDC involvement in biological effects of LMWF in HUVECs. 2D-angiogenesis (A) and migration (B) assays were performed in SDC-1-siRNA- or SDC-4-siRNA- or SNC-siRNA-transfected control cells (B, left panel), or in cells treated with specific anti-SDC-1 or anti-SDC-4 antibodies (B, right panel). Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells or isotypes. (C) SDC-1 and SDC-4 mRNA expression was analyzed in EXT2- or HPSE-siRNA-transfected cells. * p < 0.05 versus SNC-siRNA-transfected control cells or isotypes. A.U.: arbitrary unit.
Figure 8.
Assessment of SDC involvement in biological effects of LMWF in HUVECs. 2D-angiogenesis (A) and migration (B) assays were performed in SDC-1-siRNA- or SDC-4-siRNA- or SNC-siRNA-transfected control cells (B, left panel), or in cells treated with specific anti-SDC-1 or anti-SDC-4 antibodies (B, right panel). Control LMWF induction was arbitrary set at 100% for SNC-siRNA-transfected cells or isotypes. (C) SDC-1 and SDC-4 mRNA expression was analyzed in EXT2- or HPSE-siRNA-transfected cells. * p < 0.05 versus SNC-siRNA-transfected control cells or isotypes. A.U.: arbitrary unit.
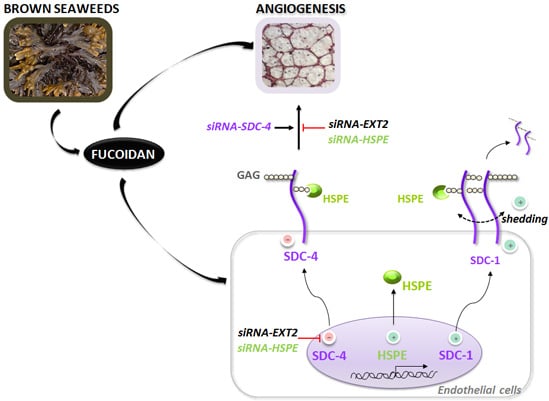
Our results could be summarized as follows: 1/LMWF increased endothelial cell migration and vascular tube formation; 2/LMWF modified HS- and SDC metabolism (increased heparanase (HPSE) level and activity, change in SDC-1/-4 expression and shedding); 3/EXT2, HPSE and SDC-4 are involved in LMWF cellular effects since silencing EXT2, HPSE, or SDC-4 affect LMWF-induced angiogenesis; 4/Our data also evidenced a link between EXT2, HPSE, and SDC-4 level since silencing EXT2 or HPSE led to increased translational expression of SDC-4.
Fucoidan as a heparin-like molecule can physically interact with several heparin-binding growth factors and chemokines. Fucoidan may promote or inhibit growth factor effects by trapping endogenously-released growth factors, or by displacing its ligands from their storage sites, increasing their bioavailability. Thus, LMWF has been shown to release the glycosaminoglycan-bound stromal cell-derived factor-1 (SDF-1)/CXCL12, which mobilizes progenitor cells [
22]. (SDF-1)/CXCL12 may participate in angiogenesis together with vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [
23]. In this context, one could suppose that the modification in HS chain synthesis or degradation would affect LMWF activities. For example, it has been demonstrated that the overall size of HS chains, as well as the specific features of HS chains, including the sulfated patterns, can affect FGF signaling activation [
24]. Our data suggest that LMWF effects depend on HS, since LMWF-mediated angiogenesis is decreased either in
EXT2 (involved in HS biosynthesis) or
HPSE (involved in HS degradation) silencing conditions. Furthermore, our data also demonstrated that LMWF increased HPSE expression and activity, and does not really affect EXT1 and EXT2 expression in endothelial cells. It is of note that LMWF treatment only slightly affects the HS level in our
in vitro condition of HUVEC culture. This could be related to the law sensitivity of the dimethyl-methylene blue (DMMB) assay.
Interestingly, the inverse relations among HS, EXT1, and HPSE expressions are observed in cancer cell models. Cancer cells with higher EXT1 expression exhibited lower HPSE expression, whereas cancer cells with lower EXT1 expression exhibited higher HPSE expression [
25]. In addition, the
EXT1 knockdown with siRNA led to up-regulation of HPSE expression and potentiation of metastatic capacity [
25]. Similarly, Huegel
et al. recently demonstrated that interfering with HS function, both with the chemical antagonist Surfen or treatment with bacterial heparitinase, up-regulated endogenous HPSE gene expression, suggesting a feedback mechanism that would result in further HS reduction and increased signaling [
26]. With our data, it suggests that a coordinated regulation of key features of HS expression (EXT enzymes and HPSE) does exist even no mechanism has been brought out yet.
Our results also highlight a more important role of EXT2 than EXT1 in LMWF-inducted angiogenesis. This could be related to the fact that these enzymes can act differently on HS biosynthesis as demonstrated by Busse
et al. [
27].
Besides, HPSE overexpression has already been involved in
in vivo angiogenesis in mice models. Homozygous transgenic mice that overexpress HPSE demonstrate both a deep reduction in the size of HS chains, as well as enhanced neovascularization of mammary ducts [
28], some conclusions that seem consonant with our observations. The overexpression of HPSE by tumors may activate tumor angiogenesis through various mechanisms in addition to promoting the release of growth factor-decorated HS fragments. HPSE has been demonstrated to be a mediator of angiogenesis by different mechanisms [
29]. HPSE promotes: 1/endothelial cell migration and degradation of the subendothelial basal lamina; 2/release of active HS-bound FGF and VEGF; 3/release of HS degradation fragments that promote FGF receptor binding, dimerization and signaling. In addition, HPSE has been demonstrated to be related to changes in the distribution of SDC-1, in particular by acting on SDC-1 ectodomain shedding [
30]. We have investigated effects of LMWF on SDC-1 and SDC-4. Both heparan sulfate proteoglycans are involved in cell migration through cell cytoskeletal rearrangement, spreading, and 2D-angiogenesis. We have demonstrated that LMWF increases endothelial cell SDC-1 expression and shedding, and has an opposite effect on the same SDC-4 features. In rat injured thoracic aorta, our recent
in vivo results demonstrate that LMWF treatment increased SDC-1 expression in the neointima layer of the injured artery, but decreased the SDC-4 expression in the neointima and media layers, therefore strengthening the
in vitro data [
10].
SDC-4, but not
SDC-1 silencing in HUVECs increases the LMWF-induced angiogenesis and cell migration, suggesting that SDC-4 expression partially counteracts LMWF effects.
Furthermore, our data also evidenced an unknown link between EXT2, HPSE, and SDC-4 level since silencing
EXT2 or
HPSE led to increased translational expression of SDC-4. In these conditions, SDC-1 expression remains unchanged. These data suggested that the amount of HS present on SDC-4 core proteins could regulate the rate of SDC-4 core protein synthesis. Similarly, Ramani
et al. recently demonstrated that HS-chains of SDC-1 regulate ectodomain shedding accompanied by a very high increase in core protein synthesis [
31].
Altogether, we hypothesize that LMWF affects SDCs shedding and expression by acting through both enzymes HPSE and matrix metalloproteinase-2 [
10], leading to change in the binding and the signaling and/or the bioavailability of heparin-binding proteins in the process of angiogenesis.