Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies
Abstract
:1. Introduction
| Product | Global Production (ton/year) | Retail Price (US$/kg) | Approximate Gross Market Value (US$ million/year) |
|---|---|---|---|
| Agars | 10,600 | 18 | 191 |
| Alginates | 30,000 | 12 | 339 |
| Carrageenans | 60,000 | 10.4 | 626 |
2. Carrageenans
2.1. Common Carageenan Sources
| Seaweed Source | Products | Main Chemical Structures | Applications | Research Conducted |
|---|---|---|---|---|
| Kappaphycus alvarezii | κ-Carrageenan | 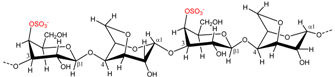 | Gelling agent (stiff and brittle gel) | [7] |
| Eucheuma spinosum | ι-Carrageenan | 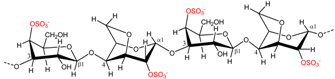 | Gelling agent (flexible soft gel) | [7] |
| Gigartina spp. Chondrus spp. | λ-Carrageenan |  | Thickener | [7] |
| Kappaphycus alvarezii | µ-Carrageenan |  | κ-Carrageenan precursor | [8] |
| Eucheuma spinosum | ν-Carrageenan | 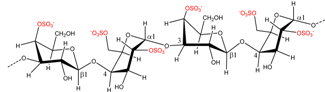 | ι-Carrageenan precursor | [8] |
| Gelidiella spp. Gelidium spp. | Agar/Agarose | 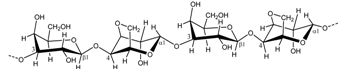 | Microbiology Gelling agent (strong and rigid) | [9] |
| Porphyra umbilicalis | Porphyran | 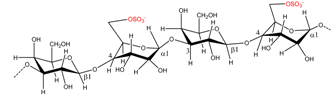 | Agar precursor | [8] |
| Laminaria spp. Sargassum spp. | Alginate |  | Gelling agent | [10,11] |
2.2. Carrageenan Chemical Structure
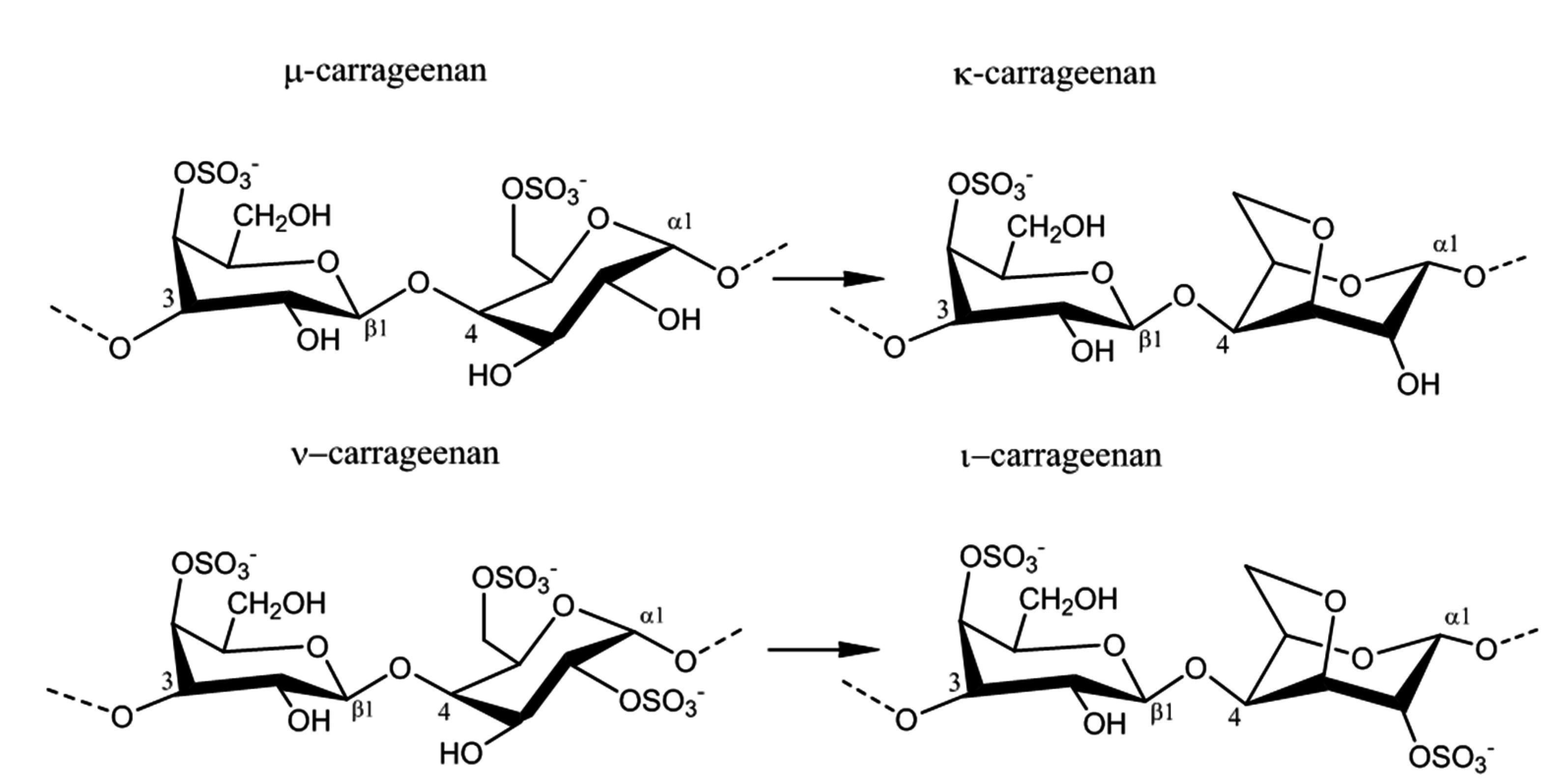
2.3. Physico-Chemical Properties of Carrageenans
2.4. Enzyme Technology for Carrageenans Extraction

2.5. Carrageenans Applications
| Hydrocolloids | Enzymes | Organisms | Catalytic Reaction | Research Conducted |
|---|---|---|---|---|
| κ-Carrageenan | κ-Carrageenase EC 3.2.1.83 GH 16 | Pseudoalteromonas carrageenovora | Endohydrolysis of (1,4)-β-d-linkages between d-galactose 4-sulfate and 3,6-anhydro-d-galactose | [29] |
| κ-Carrageenan | Sulfatase | Pseudomonas carrageenovora | Eliminates sulfate from d-galactose 4-sulfate, producing d-galactose | [37] |
| κ-Carrageenan | Carratetraose-4-O monosulfate-β-hydrolase | Pseudomonas carrageenovora | Hydrolysis of (1,4)-β-d-linkages between between d-galactose 4-sulfate and 3,6-anhydro-d-galactose in κ-carrageenan DP4 | [36] |
| κ-Carrageenan | Sulfurylase I and II | Chondrus crispus | Eliminates sulfate from d-galactose 6-sulfate of μ-carrageenan, producing 3,6 anhydro-d-galactose residues | [42] |
| ι-Carrageenan | ι-Carrageenase EC 3.2.1.157 GH 82 | Zolbellia galacta | Endohydrolysis of (1,4)-β-d-linkages between d-galactose 4-sulfate and 3,6-anhydro-d-galactose-2-sulfate | [30] |
| ι-Carrageenan | Sulfatase | Pseudoalteromonas atlantica | Eliminates sulfate from d-galactose 4-sulfate, producing d-galactose | [38] |
| ι-Carrageenan | Sulfurylases I and II | Chondrus crispus | Eliminates sulfate from d-galactose 6-sulfate of ν-carrageenan, producing 3,6 anhydro-d-galactose residues | [8] |
| λ-Carrageenan | λ-Carrageenase EC 3.2.1.162 | Pseudoalteromonas carrageenovora | Endohydrolysis of (1,4)-β-d-linkages between d-galactose 2-sulfate and d-galactose 2,6-sulfate | [31] |
| Agar | Gal-6-sulfurylase EC 2.5.1.5 | Porphyra umbilicalis | Eliminates sulfate from l-galactose 6-sulfate of porphyran, producing 3,6-l-anhydrogalactose | [43] |
| Agar | α-Agarase EC 3.2.1.158 | Thalassomonas agarivorans JAMP-A33 | Endohydrolysis of (1,3)-α-l-linkages between d-galactose and 3,6-anhydro-l-galactose | [44] |
| Agar | β-Agarase EC 3.2.1.81 | Alteromonas sp. SY37-12 | Hydrolysis of (1,4)-β-d-linkages between 3,6-anhydro-l-galactose and d-galactose in agar | [45] |
| Alginate | Mannuronate lyase EC 4.2.2.3 PL5 | Azotobacter chroococcum | Cleavage of polysaccharides with β-d-mannuronate | [46] |
| Alginate | Guluronate lyase EC 4.2.2.11 PL7 | Klebsiella aerogenes | Cleavage of polysaccharides containing α-l-guluronate | [47] |
| Alginate | Mannuronan C5 epimerase | Azotobacter vinelandii | Epimerisation of β-d-mannuronic acid residues at C5 | [48] |
3. Agars
3.1. Common Red Seaweed Sources
3.2. Chemical Structure of Agar
3.3. Physico-Chemical Properties of Agar
| Properties | Agar | Carrageenan |
|---|---|---|
| Solubility | Boiling water | Boiling water |
| Gel Strength (1.5% at 20 °C) | 700–1000 g/cm3 | 100–350 g/cm3 |
| Viscosity (1.5% at 60 °C) | 10–100 centipoise | 30–300 centipoise |
| Melting point | 85–95 °C | 50–70 °C |
| Gelling point | 32–45 °C | 30–50 °C |
3.4. Extraction and Processing of Agar
3.5. Commercial Applications of Agar
4. Alginates
4.1. Common Brown Seaweed Sources of Alginate
4.2. Chemical Structure and Physico-Chemical Properties of Alginate
4.3. Alginates Extraction and Processing
4.4. Common Applications for Alginates
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Sea Weed Site: Information on Marine Algae. Available online: http://seaweed.ie/uses_general/industrialgums.php (accessed on 18 May 2015).
- Mchugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Msuya, F. The impact of seaweed farming on the socioeconomic status of coastal communities in Zanzibar, Tanzania. World Aquac. 2011, 42, 45–48. [Google Scholar]
- McHugh, D. Production and Utilization of Products from Commercial Seaweeds; FAO Fisheries Technical Paper 288; Food and Agriculture Organization of the United Nations: Rome, Italy, 1987. [Google Scholar]
- Van De Velde, F.; Peppelman, H.A.; Rollema, H.S.; Hans, R. On the structure of κ/ι-hybrid carrageenans. Carbohydr. Res. 2001, 331, 271–283. [Google Scholar]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N. Social and Economic Dimensions of Carrageenan Seaweed Farming; Fisheries and Aquaculture Technical Paper 580; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- De Ruiter, G.A.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar]
- Genicot-Joncour, S.; Poinas, A.; Richard, O.; Potin, P.; Rudolph, B.; Kloareg, B.; Helbert, W. The cyclization of the 3,6-anhydro-galactose ring of iota-carrageenan is catalyzed by two d-galactose-2,6-sulfurylases in the red alga Chondrus crispus. Plant Physiol. 2009, 151, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.P.; Chang, L.L.; Chang, S.N.; Wang, E.C.; Hwang, L.C.; Chen, Y.H.; Wang, Y.M. Successful preparation and characterization of biotechnological grade agarose from indigenous Gelidium amansii of Taiwan. Process. Biochem. 2012, 47, 550–554. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar] [PubMed]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M.; Yaphe, W. Sulfate content of carrageenan and agar determined by infrared spectroscopy. Bot. Mar. 1986, 29, 335–340. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Craigie, J.S. Cell walls. In Biology of the Red Algae; Cole, K., Sheath, R., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 221–257. [Google Scholar]
- Montero, P.; Pe, M. Effects of Na+, K+ and Ca2+ on gels formed from fresh mince containing a carrageenan or alginate. Food Hydrocoll. 2002, 16, 375–385. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods ofpPreparation, characterisation and application. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; InTech: Rijeka, Croatia, 2011; Chapter 5. [Google Scholar]
- Rees, D. Structure, conformation and mechanism in the formation of polysaccharide gels and networks. Adv. Carbohydr. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [PubMed]
- Wu, P.; Imai, M. Novel Biopolymer Composite Membrane Involved with Selective Mass Transfer and Excellent Water Permeability; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Eng. Sci. 2012, 26, 3–9. [Google Scholar]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Alemán, A.; Gómez-Guillén, M.C.; Montero, M.P. Enzyme-assisted extraction of κ/ι-hybrid carrageenan from Mastocarpus stellatus for obtaining bioactive ingredients and their application for edible active film development. Food Funct. 2014, 5, 319. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, I.W.F.; Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; de Paula, G.A.; Lima, T.D.B.; Benevides, N.M.B. Iota-carrageenans from Solieria filiformis (Rhodophyta) and their effects in the inflammation and coagulation. Acta Sci. Technol. 2012, 34, 127–135. [Google Scholar] [CrossRef]
- Varadarajan, S.A.; Ramli, N.; Ariff, A.; Said, M.; Yasir, S.M. Development of high yielding carragenan extraction method from Eucheuma Cotonii using cellulase and Aspergillus niger. In Proceedings of Prosiding Seminar Kimia Bersama UKM-ITB VIII, Bangi, Malaysia, 9–11 Jan 2009; pp. 461–469.
- Fleurence, J.; Massiani, L.; Guyader, O.; Mabeau, S. Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 1995, 7, 393–397. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-assisted extraction of bioactive material from Chondrus crispus and Codium fragile and its effect on Herpes simplex virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potin, P.; Richard, C.; Barbeyron, T.; Henrissat, B.; Gey, C.; Petillot, Y.; Forest, E.; Dideberg, O.; Rochas, C.; Kloareg, B. Processing and hydrolytic mechanism of the cgkA-encoded κ-carrageenase of Alteromonas carrageenovora. Eur. J. Biochem. 1995, 228, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Barbeyron, T.; Michel, G.; Potin, P.; Henrissat, B.; Kloareg, B. Iota-carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J. Biol. Chem. 2000, 275, 35499–35505. [Google Scholar] [CrossRef] [PubMed]
- Guibet, M.; Barbeyron, T.; Genicot, S.; Kloareg, B.; Michel, G. Degradation of λ-carrageenan by Pseudoalteromonas carrageenovora λ-carrageenase: A new family of glycoside hydrolases unrelated to κ- and ι-carrageenases. Biochem. J. 2007, 114, 105–114. [Google Scholar]
- Lemoine, M.; Nyvall Collén, P.; Helbert, W. Physical state of kappa-carrageenan modulates the mode of action of kappa-carrageenase from Pseudoalteromonas carrageenovora. Biochem. J. 2009, 419, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Chantalat, L.; Duee, E.; Barbeyron, T.; Henrissat, B.; Kloareg, B.; Dideberg, O. The kappa-carrageenase of P. carrageenovora features a tunnel-shaped active site: A novel insight in the evolution of Clan-B glycoside hydrolases. Structure 2001, 9, 513–25. [Google Scholar] [CrossRef] [PubMed]
- Henares, B.M.; Enriquez, E.P.; Dayrit, F.M.; Rojas, N.R.L. Iota-carrageenan hydrolysis by Pseudoalteromonas carrageenovora IFO12985. Philipp. J. Sci. 2010, 139, 131–138. [Google Scholar]
- Ma, S.; Duan, G.; Chai, W.; Geng, C.; Tan, Y.; Wang, L.; Le Sourd, F.; Michel, G.; Yu, W.; Han, F. Purification, cloning, characterization and essential amino acid residues analysis of a new ι-carrageenase from Cellulophaga sp. QY3. PLoS ONE 2013, 8, e64666. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.W.; Williamson, F.B. Neocarratetraose 4-O-monosulphate P-hydrolase from Pseudomonas carrageenovora. Eur. J. Biochem. 1981, 456, 447–456. [Google Scholar] [CrossRef]
- McLean, M.W.; Williamson, F.B. Glycosulphatase from Pseudomonas carrageenovora. Eur. J. Biochem. 1979, 101, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Préchoux, A.; Genicot, S.; Rogniaux, H.; Helbert, W. Controlling carrageenan structure using a novel formylglycine-dependent sulfatase, an endo-4S-iota-carrageenan sulfatase. Mar. Biotechnol. 2013, 15, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Myette, J.R.; Shriver, Z.; Pojasek, K.; Venkataraman, G.; Sasisekharan, R. The heparin/heparan sulfate 2-O-sulfatase from Flavobacterium heparinum: A structural and biochemical study of the enzyme active site and saccharide substrate specificity. J. Biol. Chem. 2003, 278, 12167–12174. [Google Scholar] [CrossRef] [PubMed]
- Renn, D. Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.F.; Craigie, J.S. Sulfohydrolase activity and carrageenan biosynthesis in Chondrus crispus (Rhodophyceae). Plant Physiol. 1978, 61, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A. Enzymic desulphation of porphyran. Biochem. J. 1961, 80, 449–453. [Google Scholar] [PubMed]
- Ohta, Y.; Hatada, Y.; Miyazaki, M.; Nogi, Y.; Ito, S.; Horikoshi, K. Purification and Characterization of a novel a-agarase from a Thalassomonas sp. Curr. Microbiol. 2005, 50, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mou, H.; Jiang, X.; Guan, H. Characterization of a novel β-agarase from marine Alteromonas sp. SY37-12 and its degrading products. Appl. Microbiol. Biotechnol. 2006, 71, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Kodama, T. Purification and propertes of poly(β-d-mannuronate) lyase from Azotobacter chroococcum. Appl. Microbiol. Biotechnol. 1996, 44, 576–581. [Google Scholar] [CrossRef]
- Boyd, J.; Turvey, J.R. Isolation of poly-alpha-l-guluronate lyase from Klebsiella aerogenes. Carbohydr. Res. 1977, 57, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Larsen, B. Biosynthesis of Alginate: Part II. Polymannuronic acid C-5-epimerase from Azotobacter vinelandii. Carbohydr. Res. 1971, 17, 297–308. [Google Scholar]
- Rees, D.A. Enzymic synthesis of 3:6-anhydro-l-galactose within porphyran from l-galactose 6-sulphate units. Biochem. J. 1961, 81, 347–352. [Google Scholar] [PubMed]
- Araki, C.; Arai, K.; Hirase, S. Studies on the chemical constitution of agar-agar. Bull. Chem. Soc. Jpn. 1967, 40, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Bowker, D.M.; Turkey, J.R. Water-soluble polysaccharides of the red alga Laurencia pinnatifida Part I. Constituents Units. J. Chem. Soc. 1968, 1968, 983–988. [Google Scholar]
- Lahaye, M.; Yaphe, W.; Viet, M.T.P.; Rochas, C. 13C-NMR spectroscopic investigation of methylated and charged agarose oligosaccharides and polysaccharides. Carbohydr. Res. 1989, 190, 249–265. [Google Scholar] [CrossRef]
- Craigie, J.S.; Jurgens, A. Structure of agars from Gracilaria tikvahiae rhodophyta: Location of 4-O-methyl-l-galactose and sulphate. Carbohydr. Polym. 1989, 11, 265–278. [Google Scholar] [CrossRef]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. Hydrobiologia 1991, 221, 137–148. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M. Average molecular weight and molecular weight distribution of agarose and agarose-type polysaccharides. Carbohydr. Polym. 1989, 10, 289–298. [Google Scholar] [CrossRef]
- Agargel. Available online: http://www.agargel.com.br/index-en.html (accessed on 18 May 2015).
- Duff, R.B.; Perciaval, E.G. Carbohydrate Sulphuric Ester. Part II. The isolation of 3:6-anhydromethylhexosides from methylhexopyranoside sulphatases. J. Chem. Soc. 1941, 1941, 830–833. [Google Scholar] [CrossRef]
- Jam, M.; Flament, D.; Allouch, J.; Potin, P.; Thion, L.; Kloareg, B.; Czjzek, M.; Helbert, W.; Michel, G.; Barbeyron, T. The endo-beta-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: Two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem. J. 2005, 385, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Moe, S.T.; Skjåk-Bræk, G.; Smidsrød, O. Alginates. In Food Poolysaccharrides and Their Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Davis, T.A.; Ramirez, M.; Mucci, A.; Larsen, B. Extraction, isolation and cadmium binding of alginate from Sargassum spp. J. Appl. Phycol. 2004, 16, 275–284. [Google Scholar] [CrossRef]
- Chèze-Lange, H.; Beunard, D.; Dhulster, P.; Guillochon, D.; Cazé, A.M.; Morcellet, M.; Saude, N.; Junter, G.A. Production of microbial alginate in a membrane bioreactor. Enzyme Microb. Technol. 2002, 30, 656–661. [Google Scholar] [CrossRef]
- Calumpong, H.P.; Maypa, A.P.; Magbanua, M. Population and alginate yield and quality assessment of four Sargassum species in Negros Island, central Philippines. Hydrobiologia 1999, 398–399, 211–215. [Google Scholar] [CrossRef]
- Vauchel, P.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. A new process for the extraction of alginates from Laminaria digitata: Reactive extrusion. Food Bioprocess Technol. 2008, 1, 297–300. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrød, O.; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Ertesvåg, H.; Høidal, H.K.; Schjerven, H.; Svanem, B.I.; Valla, S. Mannuronan C-5-epimerases and their application for in vitro and in vivo design of new alginates useful in biotechnology. Metab. Eng. 1999, 1, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, K.; Burlot, A.S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Sharma, C. Chitosan and alginate wound dressings: A short review. Trends Biomater. Artif. Organs 2004, 18, 18–23. [Google Scholar]
- Finotelli, P.V.; da Silva, D.; Sola-Penna, M.; Rossi, A.M.; Farina, M.; Andrade, L.R.; Takeuchi, A.Y.; Rocha-Leão, M.H. Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids Surf. B Biointerfaces 2010, 81, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.K.; Cohen, D.J.; Sedlaczek, J.; Pinsker, E.J.; Boyan, B.D.; Schwartz, Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials 2013, 34, 8172–8184. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Llanes, F.; Volesky, B.; Mucci, A. Metal selectivity of Sargassum spp. and their alginates in relation to their a-l-guluronic acid content and conformation. Environ. Sci. Technol. 2003, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340-3359. https://doi.org/10.3390/md13063340
Rhein-Knudsen N, Ale MT, Meyer AS. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Marine Drugs. 2015; 13(6):3340-3359. https://doi.org/10.3390/md13063340
Chicago/Turabian StyleRhein-Knudsen, Nanna, Marcel Tutor Ale, and Anne S. Meyer. 2015. "Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies" Marine Drugs 13, no. 6: 3340-3359. https://doi.org/10.3390/md13063340
APA StyleRhein-Knudsen, N., Ale, M. T., & Meyer, A. S. (2015). Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Marine Drugs, 13(6), 3340-3359. https://doi.org/10.3390/md13063340






