1H NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea
Abstract
:1. Introduction
2. Results and Discussion
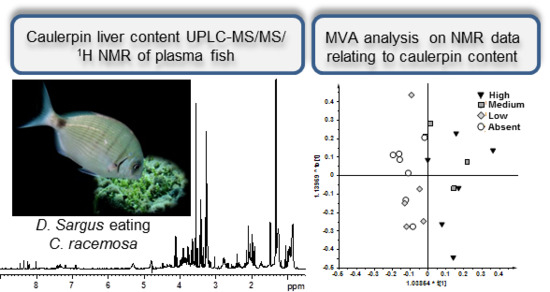
2.1. NMR Spectroscopy and MVA (Multivariate Analysis)
| Caulerpin (μg/g) | Caulerpin (μg/g) | Caulerpin (μg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| June | October | June | October | June | October | ||||||
| PC1a | 3.72 | PC1b | 0.00 | BR1a | 7.41 | BR1b | 29.09 | TG1a | 0.00 | TG1b | 10.07 |
| PC2a | 3.98 | PC2b | 0.00 | BR2a | 8.78 | BR2b | 3.2 | TG2a | 0.00 | TG2b | 0.00 |
| PC3a | 5.09 | PC3b | 0.00 | BR3a | 7.07 | BR3b | 8.8 | TG3a | 0.00 | TG3b | 27.94 |
| PC4a | 31.43 | PC4b | 0.00 | BR4a | 54.58 | BR4b | 28.93 | TG4a | 4.6 | TG4b | 78.33 |
| PC5a | 29.07 | PC5b | 0.00 | BR5a | 43.82 | BR5b | 54.6 | TG5a | 6.72 | TG5b | 39.02 |
| - | PC6b | 0.00 | BR6a | 30.8 | BR6b | 5.5 | - | TG6b | 40.98 | ||
| - | PC7b | 0.00 | - | BR7b | 114.42 | - | TG7b | 93.6 | |||
| - | PC8b | 6.34 | - | BR8b | 235.75 | - | TG8b | 114.08 | |||
| - | PC9b | 55.02 | |||||||||
| mean | 14.66 | 6.82 | 25.41 | 60.04 | 2.26 | 50.5 | |||||
| sem | 6.38 | 5.72 | 8.48 | 28.23 | 1.43 | 14.39 | |||||

| Metabolites | δ (ppm) |
|---|---|
| cholesterol | 0.74 (m a, CH3) |
| lipoprotein | 0.83 (t), 0.85 (t), 0.86 (t), 0.88 (t), 0.93 (t), 0.98 (t), 1.05 (t) (CH3CHn) |
| leucine | 0.96 (d, CH3), 0.98 (d, CH3), 1.72 (m, CH), 1.73 (m, CH) |
| isoleucine | 0.94 (t, CH3), 1.02 (d, CH3) |
| valine | 1.00 (d, CH3), 1.05 (d, CH3) |
| lactate | 1.33 (d, CH3), 4.11, (q, CH) |
| alanine | 1.48 (d, CH3), 3.80 (m, CH) |
| lipoprotein | 1.20 (m), 1.25 (m), 1.26 (m), 1.31 (m) (CH2)n |
| acetate | 1.94 (s, CH3) |
| lipid | 1.51 (m), 1.55 (m) (CH2CH2CO) |
| lipid | 1.44, 1.52, 1.68 (m) (CH2CH2C=C) |
| lipid | 1.98, 2.04, 2.08, 2.14, 2.19 (CH2C=C) |
| lipid | 2.23 (m), 2.35, 2.42, 2.46, (CH2CO) |
| lipid | 2.65, 2.69, 2.76, 2.77, 2.78 (m) (C=CCH2C=C) |
| lipid | 5.31 (m) (HC=CH) |
| methionine | 2.14 (s, S-CH3), 2.66 (t, CH2), 3.80 (t, CH) |
| glutamate | 2.07 (m, CH2), 2.35 (m, CH2) |
| glutamine | 2.14 (m, CH2), 2.45 (m, CH2) |
| citrate | 2.54 (d, CH2), 2.67 (d, CH2) |
| choline | 3.24 (s, N(CH3)3) |
| TMAO | 3.27 (s, N(CH3)3) |
| betaine | 3.27 (s, N(CH3)3), 3.91 (s, CH2) |
| taurine | 3.27 (t, CH2NH), 3.42 (t, CH2SO3) |
| glycine | 3.59 (s, CH) |
| glycerol | 3.56 (dd, CH2), 3.66 (dd, CH2), 3.89 (m, CH) |
| α-glucose | 5.24 (d, CH) |
| β-glucose | 4.65 (d, CH) |
| tyrosine | 6.91 (m, C3,5H ring), 7.20 (m, C2,6H ring) |
| phenylalanine | 7.43 (m, C3,5H), 7.38 (m, C4H), 7.33 (m, C2,6H) |
| hypoxanthine | 8.20 (s, C2H), 8.21 (s, C8H) |
| adenosine | 8.24 (s, C2H), 8.35 (s, C8H) |
| formate | 8.46 (s, CH) |




2.2. Discussion
3. Experimental Section
3.1. Sample Collection
3.2. NMR Measurement
3.3. Data Processing
3.4. Multivariate Statistical Analyses
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Byrnes, J.E.; Reynolds, P.L.; Stachowicz, J.J. Invasions and extinctions reshape coastal marine food webs. PLoS ONE 2007, 2, e295. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Çinar, M.E.; Garcia Raso, J.; Bianchi, C.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s marine strategy framework directive (MSFD). Part I. spatial distribution. Medit. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D. The spread of Caulerpa racemosa var. cylindracea in the Mediterranean sea: An example of how biological invasions can influence beta diversity. Mar. Environ. Res. 2008, 65, 50–61. [Google Scholar]
- Katsanevakis, S.; Issaris, Y.; Poursanidis, D.; Thessalou-Legaki, M. Vulnerability of marine habitats to the invasive green alga Caulerpa racemosa var. cylindracea within a marine protected area. Mar. Environ. Res. 2010, 70, 210–218. [Google Scholar]
- Bulleri, F.; Cristaudo, C.; Alestra, T.; Benedetti-Cecchi, L. Crossing gradients of consumer pressure and physical stress on shallow rocky reefs: A test of the stress-gradient hypothesis. J. Ecol. 2011, 99, 335–344. [Google Scholar] [CrossRef]
- Felline, S.; Caricato, R.; Cutignano, A.; Gorbi, S.; Lionetto, M.G.; Mollo, E.; Regoli, F.; Terlizzi, A. Subtle effects of biological invasions: Cellular and physiological responses of fish eating the exotic pest Caulerpa racemosa. PLoS ONE 2012, 7, e38763. [Google Scholar] [CrossRef] [PubMed]
- Felline, S.; Mollo, E.; Ferramosca, A.; Zara, V.; Regoli, F.; Gorbi, S.; Terlizzi, A. Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar. Biol. 2014, 161, 1275–1283. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Mollo, E.; Cimino, G.; Ghiselin, M.T. Alien biomolecules: a new challenge for natural product chemists. Biol. Invasions 2015, 17, 941–950. [Google Scholar] [CrossRef]
- Raniello, R.; Mollo, E.; Lorenti, M.; Gavagnin, M.; Buia, M.C. Phytotoxic activity of caulerpenyne from the Mediterranean invasive variety of Caulerpa racemosa: A potential allelochemical. Biol. Invasions 2007, 9, 361–368. [Google Scholar] [CrossRef]
- Rocha, F.D.; Soares, A.R.; Houghton, P.J.; Pereira, R.C.; Kaplan, M.A.C.; Teixeira, V.L. Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytother. Res. 2007, 21, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Box, A.; Deudero, S.; Sureda, A.; Blanco, A.; Alòs, J.; Terrados, J.; Grau, A.M.; Riera, F. Diet and physiological responses of Spondyliosoma cantharus (Linnaeus, 1758) to the Caulerpa racemosa var. cylindracea invasion. J. Exp. Mar. Biol. Ecol. 2009, 380, 11–19. [Google Scholar] [CrossRef]
- Terlizzi, A.; Felline, S.; Lionetto, M.G.; Caricato, R.; Perfetti, V.; Cutignano, A.; Mollo, E. Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat. Biol. 2011, 12, 109–117. [Google Scholar] [CrossRef]
- Gorbi, S.; Giuliani, M.; Pittura, L.; d’Errico, G.; Terlizzi, A.; Felline, S.; Grauso, L.; Mollo, E.; Cutignano, A.; Regoli, F. Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar. Environ. Res. 2014, 96, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Solanky, K.S.; Burton, I.W.; MacKinnon, S.L.; Walter, J.A.; Dacanay, A. Metabolic changes in Atlantic salmon exposed to Aeromonas salmonicida detected by 1H-nuclear magnetic resonance spectroscopy of plasma. Dis. Aquat. Org. 2005, 65, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Karakach, T.K.; Huenupi, E.C.; Soo, E.C.; Walter, J.A.; Afonso, L.O. 1H-NMR and mass spectrometric characterization of the metabolic response of juvenile Atlantic salmon (Salmo salar) to long-term handling stress. Metabolomics 2009, 5, 123–137. [Google Scholar] [CrossRef]
- Samuelsson, L.M.; Björlenius, B.; Förlin, L.; Larsson, D.J. Reproducible 1H NMR-based metabolomic responses in fish exposed to different sewage effluents in two separate studies. Environ. Sci. Technol. 2011, 45, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, L.M.; Förlin, L.; Karlsson, G.; Adolfsson-Erici, M.; Larsson, D.J. Using NMR metabolomics to identify responses of an environmental estrogen in blood plasma of fish. Aquat. Toxicol. 2006, 78, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Foxall, P.J.; Spraul, M.; Farrant, R.D.; Lindon, J.C. 750 MHZ 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal. Chem. 1995, 67, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Mannina, L.; Sobolev, A.P.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.Y.; Purcell, E.M. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev. 1954, 94, 630. [Google Scholar] [CrossRef]
- Mallol, R.; Rodriguez, M.A.; Brezmes, J.; Masana, L.; Correig, X. Human serum/plasma lipoprotein analysis by NMR: Application to the study of diabetic dyslipidemia. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 70, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wikström, C.; Albano, C.; Eriksson, L.; Fridén, H.; Johansson, E.; Nordahl, A.; Rannar, S.; Sandberg, M.; Kettaneh-Wold, N.; Wold, S. Multivariate process and quality monitoring applied to an electrolysis process: Part I. Process supervision with multivariate control charts. Chemometr. Intell. Lab. 1998, 42, 221–231. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Tamburello, L.; Benedetti-Cecchi, L.; Masini, L.; Bulleri, F. Habitat heterogeneity promotes the coexistence of exotic seaweeds. Oecologia 2013, 172, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Verlaque, M. The Caulerpa racemosa invasion: A critical review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E. The Handbook of Metabonomics and Metabolomics, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2007; p. 561. [Google Scholar]
- Teague, C.R.; Dhabhar, F.S.; Barton, R.H.; Beckwith-Hall, B.; Powell, J.; Cobain, M.; Singer, B.; McEwen, B.S.; Lindon, J.C.; Nicholson, J.K. Metabonomic studies on the physiological effects of acute and chronic psychological stress in sprague-dawley rats. J. Proteome Res. 2007, 6, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Takeuchi, T.; Yokoyama, M.; Seikai, T. Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus. Fish. Sci. 2002, 68, 824–829. [Google Scholar] [CrossRef]
- Huxtable, R. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [PubMed]
- Lombardini, J.; Pang, P.K.; Griffith, R.W. Amino acids and taurine in intracellular osmoregulation in marine animals. Occas. Pap. Calif. Acad. Sci. 1979, 134, 160–171. [Google Scholar]
- Yokoyama, M.; Kaneniwa, M.; Sakaguchi, M. Metabolites of l-(35S) cysteine injected into the peritoneal cavity of rainbow trout. Fish. Sci. 1997, 63, 799–801. [Google Scholar]
- Gaylord, T.G.; Teague, A.M.; Barrows, F.T. Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2006, 37, 509–517. [Google Scholar] [CrossRef]
- Lunger, A.N.; McLean, E.; Gaylord, T.; Kuhn, D.; Craig, S. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Shamushaki, V.A.J.; Kasumyan, A.O.; Abedian, A.; Abtahi, B. Behavioural responses of the persian sturgeon (Acipenser persicus) juveniles to free amino acid solutions. Mar. Freshw. Behav. Physiol. 2007, 40, 219–224. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.A.; Ewart, K.V.; Short, C.E.; Burton, I.W.; Driedzic, W.R. Accelerated hepatic glycerol synthesis in rainbow smelt (Osmerus mordax) is fuelled directly by glucose and alanine: A 1H and 13C nuclear magnetic resonance study. J. Exp. Zool. Part A Comp. Exp. Biol. 2006, 305, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, S.; Pandey, K.C.; Gopal, K. Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic. Biochem. Physiol. 2007, 88, 268–272. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Cinelli, F. Threat to macroalgal diversity: Effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar. Ecol. Prog. Ser. 2001, 210, 149–159. [Google Scholar] [CrossRef]
- Terlizzi, A.; Fraschetti, S.; Guidetti, P.; Boero, F. The effects of sewage discharge on shallow hard substrate sessile assemblages. Mar. Pollut. Bull. 2002, 44, 544–550. [Google Scholar] [CrossRef]
- Fraschetti, S.; Terlizzi, A.; Bussotti, S.; Guarnieri, G.; D’Ambrosio, P.; Boero, F. Conservation of Mediterranean seascapes: Analyses of existing protection schemes. Mar. Environ. Res. 2005, 59, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, Å.M.; Wheelock, C.E. Trials and tribulations of omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar]
- Loo, R.L.; Coen, M.; Ebbels, T.; Cloarec, O.; Maibaum, E.; Bictash, M.; Yap, I.; Elliott, P.; Stamler, J.; Nicholson, J.K. Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. Anal. Chem. 2009, 81, 5119–5129. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.; Chan, Q.; Ebbels, T.; de Iorio, M.; Brown, I.J.; Veselkov, K.A. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pascali, S.A.; Del Coco, L.; Felline, S.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. 1H NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea. Mar. Drugs 2015, 13, 3550-3566. https://doi.org/10.3390/md13063550
De Pascali SA, Del Coco L, Felline S, Mollo E, Terlizzi A, Fanizzi FP. 1H NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea. Marine Drugs. 2015; 13(6):3550-3566. https://doi.org/10.3390/md13063550
Chicago/Turabian StyleDe Pascali, Sandra A., Laura Del Coco, Serena Felline, Ernesto Mollo, Antonio Terlizzi, and Francesco P. Fanizzi. 2015. "1H NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea" Marine Drugs 13, no. 6: 3550-3566. https://doi.org/10.3390/md13063550
APA StyleDe Pascali, S. A., Del Coco, L., Felline, S., Mollo, E., Terlizzi, A., & Fanizzi, F. P. (2015). 1H NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea. Marine Drugs, 13(6), 3550-3566. https://doi.org/10.3390/md13063550