Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia
Abstract
:1. Introduction
2. Results
2.1. Effects of MCPs on Body Weight, and Physiological and Neurobehavioral Development Indexes of Male Rats with PA

| Groups | N | Pinnae Detachment (day) | Incisor Eruption (day) | Eyelid Separation (day) | Testicular Descent (day) |
|---|---|---|---|---|---|
| CD control | 15 | 3.500 ± 0.378 | 11.000 ± 0.267 | 14.062 ± 0.177 | 20.125 ± 0.232 |
| PA control | 15 | 4.000 ± 0.267 ** | 11.938 ± 0.678 ** | 15.062 ± 0.496 ** | 20.562 ± 0.417 * |
| 0.33 g/kg | 15 | 3.875 ± 0.232 * | 11.750 ± 0.463 ** | 14.812 ± 0.594 ** | 20.812 ± 0.458 ** |
| 1.0 g/kg | 15 | 3.938 ± 0.177 ** | 11.625 ± 0.354 * | 14.750 ± 0.535 * | 20.750 ± 0.463 ** |
| 3.0 g/kg | 15 | 3.812 ± 0.259 * | 11.562 ± 0.417 * | 14.688 ± 0.530 * | 20.625 ± 0.354 * |
| Groups | N | Surface Righting (day) | Negative Geotaxis (day) | Cliff Avoidance (day) |
|---|---|---|---|---|
| CD control | 15 | 6.000 ± 0.3120 | 7.000 ± 0.378 | 7.062 ± 0.177 |
| PA control | 15 | 6.850 ± 0.5126 ** | 7.669 ± 0.317 ** | 7.631 ± 0.344 ** |
| 0.33 g/kg | 15 | 6.613 ± 0.3610 * | 7.562 ± 0.393 * | 7.625 ± 0.354 ** |
| 1.0 g/kg | 15 | 6.574 ± 0.3932 * | 7.500 ± 0.380 * | 7.582 ± 0.397 * |
| 3.0 g/kg | 15 | 6.562 ± 0.4816 * | 7.427 ± 0.397 * | 7.490 ± 0.373 * |
2.2. Effect of MCPs on the Performance in Behavioral Tests of Male Rats with PA
2.2.1. MCPs Intervention Did Not Significantly Impact the Locomotion of Male Rats with PA in the Open-Field Test
| Groups | N | Time Spent in Central Grids (s) | Number of Grid Crossing | Frequency of Rearing | Frequency of Grooming |
|---|---|---|---|---|---|
| CD control | 15 | 1.50 ± 0.535 | 57.00 ± 16.449 | 15.62 ± 6.906 | 1.88 ± 0.835 |
| PA control | 15 | 3.15 ± 1.309 * | 74.25 ± 14.260 * | 21.25 ± 5.874 | 3.12 ± 0.835 ** |
| 0.33 g/kg | 15 | 3.12 ± 1.553 * | 76.38 ± 12.512 ** | 21.62 ± 4.406 * | 3.00 ± 0.535 ** |
| 1.0 g/kg | 15 | 3.12 ± 1.885 * | 78.75 ± 11.260 ** | 22.15 ± 5.365 * | 3.00 ± 0.926 ** |
| 3.0 g/kg | 15 | 3.14 ± 0.886 * | 78.12 ± 13.674 ** | 21.75 ± 6.112 * | 3.00 ± 0.756 ** |
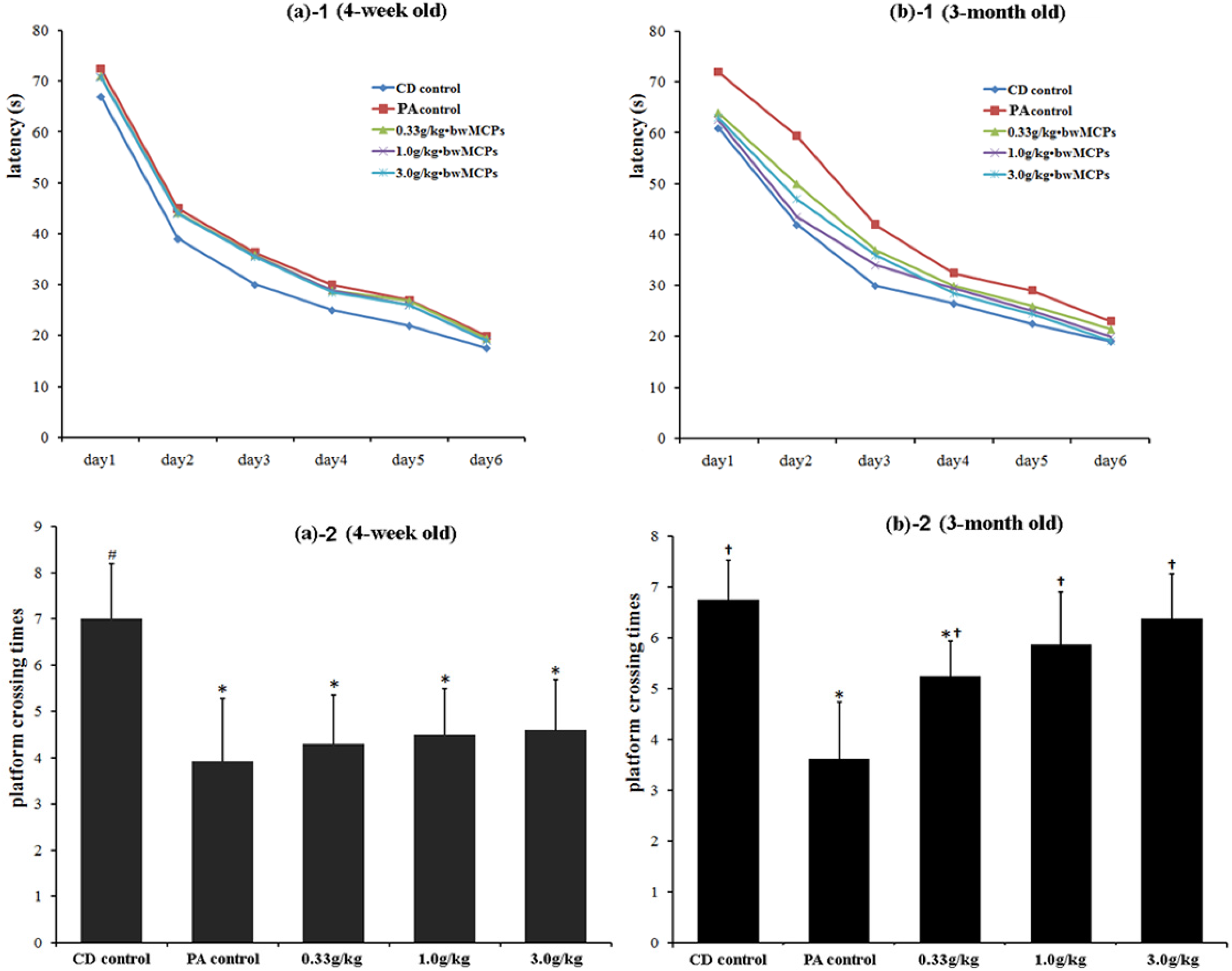
2.2.2. MCPs Improved the Long-Term Spatial Memory of Male Rats with PA in the Morris Water Maze Test
2.3. MCPs Attenuate Neuronal Loss in the Hippocampus
| Groups | N | CA1 | CA3 | DG |
|---|---|---|---|---|
| CD control | 5 | 111.17 ± 7.111 †† | 54.33 ± 2.503 | 330.75 ± 12.703 |
| PA control | 5 | 91.33 ± 4.844 ** | 48.33 ± 3.077 | 321.71 ± 22.874 |
| 0.33 g/kg | 5 | 96.29 ± 3.302 ** | 49.14 ± 4.562 | 323.88 ± 10.190 |
| 1.0 g/kg | 5 | 105.71 ± 7.158 †† | 51.43 ± 5.062 | 331.38 ± 21.421 |
| 3.0 g/kg | 5 | 106.67 ± 9.110 †† | 53.00 ± 4.873 | 331.00 ± 22.829 |
2.4. MCPs Increased the Activity of Superoxide Dismutase (SOD), But Decreased the Level of Methane Dicarboxylic Aldehyde (MDA) in the Cerebrospinal Fluid of Male Rats with PA

2.5. MCPs did not Change Brain/Body Weight Ratio, but Decreased Acetylcholinesterase (AChE) Activity in the Hippocampus of Male Rats with PA

2.6. Hippocampal Expression of cAMP Response Element Binding Protein (CREB), Phosphorylated CREB (p-CREB) and Brain-Derived Neurotrophic Factor (BDNF)
3. Discussion

4. Experimental Section
4.1. Preparation of MCPs
| Amino Acid | No. Residues per 100 Residues |
|---|---|
| Glycine | 23.77 |
| Glutamic acid | 12.22 |
| Proline | 9.79 |
| Hydroxyproline | 7.51 |
| Aspartic acid | 7.29 |
| Alanine | 6.59 |
| Arginine | 6.08 |
| Lysine | 5.66 |
| Leucine | 4.64 |
| Serine | 4.23 |
| Valine | 2.94 |
| Isoleucine | 2.57 |
| Threonine | 2.53 |
| Phenylalanine | 2.51 |
| Histidine | 1.61 |
| Methionine | 0.03 |
| Tyrosine | 0.03 |
4.2. Animals
4.3. Induction of PA
4.4. MCPs Intervention
4.5. Physiological Development of Pups
4.6. Neurobehavioral Development of Pups
4.6.1. Surface Righting Reflex
4.6.2. Negative Geotaxis Reflex
4.6.3. Cliff Avoidance Reflex
4.7. Behavioral Tests
4.7.1. Open Field Test
4.7.2. Morris Water Maze Test
4.8. Neuronal Density in the Hippocampus
4.9. Measurement of Oxidation Indexes in the Cerebrospinal Fluid
4.10. Measurement of AChE Activity in the Hippocampus
4.11. Western Blotting
4.12. Statistics Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hack, M.; Fanaro, A.A. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin. Neonatol. 2000, 5, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Addington, A.M.; Frangou, S.; Psych, M.R. The neurodevelopmental model of schizophrenia: Update 2005. Mol. Psychiatry 2005, 10, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Cutuli, D.; Chiarotti, F.; Calamandrei, G. C-section birth per se or followed by acute global asphyxia altered emotional behaviour in neonate and adult rats. Behav. Brain Res. 2006, 168, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Boksa, P.; El-Khodor, B.F. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: Possible implications for schizophrenia and other disorders. Neurosci. Biobehav. Rev. 2003, 27, 91–101. [Google Scholar] [CrossRef]
- Berger, R.; Garnier, Y. Perinatal brain injury. J. Perinat. Med. 2000, 28, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Perinatal brain injury: From pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 56–64. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Hagberg, H. Hypoxia-ischemia in the immature brain. J. Exp. Biol. 2004, 207, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Bustamante, D.; Espina-Marchant, P.; Neira-Peña, T.; Gutiérrez-Hernández, M.A.; Allende-Castro, C.; Rojas-Mancilla, E. Pathophysiology of perinatal asphyxia: Can we predict and improve individual outcomes? EPMA J. 2011, 2, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef]
- McLay, R.N.; Pan, W.; Kastin, A.J. Effects of peptides on animal and human behavior: A review of studies published in the first twenty years of the journal Peptides. Peptides 2001, 22, 2181–2255. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Rawat, D.S.; Joshi, M.C.; Joshi, P.; Atheaya, H. Marine peptides and related compounds in clinical trial. Anticancer Agents Med. Chem. 2006, 6, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Pei, X.; Yang, R.; Zhang, Z.; Gao, L.; Wang, J.; Xu, Y.; Zhao, M.; Han, X.; Liu, Z.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010, 118, 333–340. [Google Scholar] [CrossRef]
- Du Plessis, A.J.; Volpe, J.J. Perinatal brain injury in the preterm and term newborn. Curr. Opin. Neurol. 2002, 15, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, E.; Chen, Y.; Engidawork, E.; Andersson, K.; Lubec, G.; Luthman, J.; Herrera-Marschitz, M. Delayed neuronal death following perinatal asphyxia in rat. Exp. Brain Res. 1997, 115, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Cebral, E.; Loidl, C.F. Changes in neostriatal and hippocampal synaptic densities in perinatal asphyctic male and female young rats: Role of hypothermia. Brain Res. Bull. 2011, 84, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Boutaybi, N.; Razenberg, F.; Smits-Wintjens, V.E.; van Zwet, E.W.; Rijken, M.; Steggerda, S.J.; Lopriore, E. Neonatal thrombocytopenia after perinatal asphyxia treated with hypothermia: A retrospective case control study. Int. J. Pediatr. 2014, 2014, 760654. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marschitz, M.; Morales, P.; Leyton, L.; Bustamante, D.; Klawitter, V.; Espina-Marchant, P.; Allende, C.; Lisboa, F.; Cunich, G.; Jara-Cavieres, A.; et al. Perinatal asphyxia: Current status and approaches towards neuroprotective strategies, with focus on sentinel proteins. Neurotox. Res. 2011, 19, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.E.; Calzolari, S.; Molinari, M.; Iuvone, L.; Calimici, R. Neonatal anoxia induces transitory hyperactivity, permanent spatial memory deficits and CA1 cell density reduction in developing rats. Behav. Brain Res. 1991, 45, 125–134. [Google Scholar] [CrossRef]
- Iuvone, L.; Geloso, M.C.; Dell’Anna, E. Changes in open field behavior, spatial memory, and hippocampal parvalbumin immunoreactivity following enrichment in rats exposed to neonatal anoxia. Exp. Neurol. 1996, 139, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Simola, N.; Bustamante, D.; Lisboa, F.; Fiedler, J.; Gebicke-Haerter, P.J.; Morelli, M.; Tasker, R.A.; Herrera-Marschitz, M. Nicotinamide prevents the long-term effects of perinatal asphyxia on apoptosis, non-spatial working memory and anxiety in rats. Exp. Brain Res. 2010, 202, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Galeano, P.; Blanco Calvo, E.; Madureira de Oliveira, D.; Cuenya, L.; Kamenetzky, G.V.; Mustaca, A.E.; Barreto, G.E.; Giraldez-Alvarez, L.D.; Milei, J.; Capani, F. Long-lasting effects of perinatal asphyxia on exploration, memory and incentive downshift. Int. J. Dev. Neurosci. 2011, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Strackx, E.; van den Hove, D.L.; Prickaerts, J.; Zimmermann, L.; Steinbusch, H.W.; Blanco, C.E.; Gavilanes, A.W.; Vles, J.S. Fetal asphyctic preconditioning protects against perinatal asphyxia-induced behavioral consequences in adulthood. Behav. Brain Res. 2010, 208, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Capani, F.; Saraceno, G.E.; Botti, V.; Aon-Bertolino, L.; de Oliveira, D.M.; Barreto, G.; Galeano, P.; Giraldez-Alvarez, L.D.; Coirini, H. Protein ubiquitination in postsynaptic densities after hypoxia in rat neostriatum is blocked by hypothermia. Exp. Neurol. 2009, 219, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Loidl, C.F.; Gavilanes, A.W.; Van Dijk, E.H.; Vreuls, W.; Blokland, A.; Vles, J.S.; Steinbusch, H.W.; Blanco, C.E. Effects of hypothermia and gender on survival and behavior after perinatal asphyxia in rats. Physiol. Behav. 2000, 68, 263–269. [Google Scholar] [CrossRef]
- Cassel, J.C.; Cassel, S.; Galani, R.; Kelche, C.; Will, B.; Jarrard, L. Fimbria-fornix vs. selective hippocampal lesions in rats: Effects on locomotor activity and spatial learning and memory. Neurobiol. Learn. Memory 1998, 69, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Spasojevic, S.D.; Stojanovic, V.D.; Barisic, N.A.; Doronjski, A.R.; Zikic, D.R.; Babovic, S.M. Neuroprotective effects of hypothermia and erythropoietin after perinatal asphyxia in newborn rats. J. Matern. Fetal Neonatal Med. 2013, 26, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, G.E.; Bertolino, M.L.; Galeano, P.; Romero, J.I.; Garcia-Segura, L.M.; Capani, F. Estradiol therapy in adulthood reverses glial and neuronal alterations caused by perinatal asphyxia. Exp. Neurol. 2010, 223, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Fiedler, J.L.; Andrés, S.; Berrios, C.; Huaiquín, P.; Bustamante, D.; Cardenas, S.; Parra, E.; Herrera-Marschitz, M. Plasticity of hippocampus following perinatal asphyxia: Effects on postnatal apoptosis and neurogenesis. J. Neurosci. Res. 2008, 86, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Fiedler, J.L.; Andrés, S.; Berrios, C.; Huaiquín, P.; Bustamante, D.; Cardenas, S.; Parra, E.; Herrera-Marschitz, M. Oxidative stress in perinatal asphyxia. Pediatr. Neurol. 2008, 38, 181–185. [Google Scholar]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Su, G.; Ren, J.; Gu, L.; You, L.; Zhao, M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J. Agric. Food Chem. 2010, 60, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Park, P.J.; Ito, H. Purification and characterization of antioxidative peptides from bovine skin. J. Biochem. Mol. Biol. 2001, 34, 219–224. [Google Scholar]
- Ao, J.; Li, B. Amino acid composition and antioxidant activities of hydrolysates and peptide fractions from porcine collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Filgueiras, C.C.; Manhães, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Tsim, K.; Soreq, H. Acetylcholinesterase: Old questions and new developments. Front. Mol. Neurosci. 2013, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, R. CREB: A message to remember. Cell. Mol. Life Sci. 1999, 55, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, H.A.; Nelson, M.E.; Granholm, A.C. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiol. Aging 2003, 24, 37–48. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Huie, J.R.; Ying, Z.; Ferguson, A.R.; Crown, E.D.; Baumbauer, K.M.; Edgerton, V.R.; Grau, J.W. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 2007, 148, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, X.; Li, Y. Effect of marine collagen peptides on long bone development in growing rats. J. Sci. Food Agric. 2010, 90, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kimura, S.; Yazawa, T.; Endo, N. Cerebrospinal fluid sampling by lumbar puncture in rats—Repeated measurements of nitric oxide metabolites. J. Neurosci. Methods 2005, 145, 89–95. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Mar. Drugs 2015, 13, 3653-3671. https://doi.org/10.3390/md13063653
Xu L, Dong W, Zhao J, Xu Y. Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Marine Drugs. 2015; 13(6):3653-3671. https://doi.org/10.3390/md13063653
Chicago/Turabian StyleXu, Linlin, Wenhong Dong, Jie Zhao, and Yajun Xu. 2015. "Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia" Marine Drugs 13, no. 6: 3653-3671. https://doi.org/10.3390/md13063653
APA StyleXu, L., Dong, W., Zhao, J., & Xu, Y. (2015). Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Marine Drugs, 13(6), 3653-3671. https://doi.org/10.3390/md13063653






