Thermo-Acidic Pretreatment of Beach Macroalgae from Rügen to Optimize Biomethane Production—Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management
Abstract
:1. Introduction
1.1. Terrestrial Energy Crops for Biogas Production
1.2. Anaerobic Digestion of Seaweeds
1.3. Marine Biomass from Eutrophication-Afflicted Areas
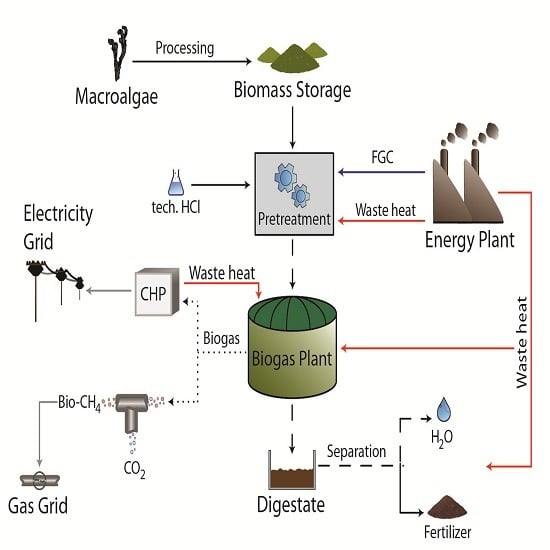
1.4. Aims of the Study
2. Results
2.1. Composition of Rügen-Mix and Theoretical Methane Potential
| Category | Element | Rügen-Mix | Maize [41,42] | Unit |
|---|---|---|---|---|
| Heavy metals (BioAbfV) | Lead (Pb) | 8.6 | 2 | mg·kg−1 TS |
| Cadmium (Cd) | 3.2 | 0.7 | ||
| Chromium (Cr) | 13 | 0.5 | ||
| Copper (Cu) | 20 | 4.5–5 | ||
| Nickel (Ni) | 15 | 5 | ||
| Mercury (Hg) | 0.05 | |||
| Zinc (Zn) | 141 | 35–56 | ||
| Macronutrients | Phosphorous (P) | 1900 | 2200 | |
| Potassium (K) | 11,400 | 17,800 | ||
| Magnesium (Mg) | 6030 | 2700 | ||
| Calcium (Ca) | 16,200 | 4500 | ||
| Sulfur (S) | 19,800 | 2700 | ||
| C/N ratio | 8.75:1 | ~30:1 | ||
| Total carbon | 35 | 43 | % TS | |
| Total nitrogen | 40,000 | 14,000 | mg·kg−1 TS | |
| Micronutrients | Molybdenum (Mo) | 1.2 | 0.3 | mg·kg−1 TS |
| Iron (Fe) | 6200 | 184 | ||
| Cobalt (Co) | 1.2 | 65 | ||
| Selenium (Se) | 0.8 | |||
| Manganese (Mn) | 180 | 29 |
| Fraction | Component | Share [%] | Theoretical CH4 | Unit |
|---|---|---|---|---|
| Volatile solids | 68.9 | |||
| Carbohydrate | 44.3 | 164 | mL·g−1 TS | |
| 238 | mL·g−1 VS | |||
| Fiber | 9.7 | |||
| Protein | 24.4 | 112 | mL·g−1 TS | |
| 163 | mL·g−1 VS | |||
| Lipid | 0.2 | 2 | mL·g−1 TS | |
| 3 | mL·g−1 VS | |||
| Inorganic solids | 31.1 | |||
| Total | 100 | 278 | mL·g−1 TS | |
| 404 | mL·g−1 VS |
2.2. BMP of Acid Hydrolysis Pretreated Rügen-Mix


| Name | Medium | PT conditions | K (d−1) | T50 | T70 | T90 | BMP [mL·g−1 VS] | SD |
|---|---|---|---|---|---|---|---|---|
| U | RM untreated | - | 0.2145 | 3.7 | 6.4 | 12.3 | 87 | ±7 |
| RM-U | RM untreated | - | 0.1912 | 3.6 | 6.3 | 12.0 | 96 | ±4 |
| 0.05 M | 0.05 M HCl | 80 °C/2 h | 0.2899 | 2.4 | 4.2 | 7.9 | 66 | ±6 |
| 0.1 M | 0.1 M HCl | 80 °C/2 h | 0.2591 | 2.7 | 4.6 | 8.9 | 95 | ±10 |
| 0.2 M | 0.2 M HCl | 80 °C/2 h | 0.2476 | 2.8 | 4.9 | 9.3 | 98 | ±11 |
| 100 °C | 0.2 M HCl | 100 °C/2 h | 0.305 | 2.8 | 4.9 | 9.4 | 103 | ±10 |
| 30 min | 0.2 M HCl | 80 °C /30 min | 0.2662 | 2.6 | 4.5 | 8.6 | 90 | ±20 |
| 60 min | 0.2 M HCl | 80 °C/60 min | 0.2536 | 2.7 | 4.7 | 9.1 | 94 | ±8 |
| 90 min | 0.2 M HCl | 80 °C/90 min | 0.2442 | 2.8 | 4.9 | 9.4 | 121 | ±7 |
| HCl | HCl pH 1.2 | 80 °C/2 h | 0.2467 | 2.8 | 4.9 | 9.3 | 103 | ±10 |
| FGC | FGC pH 1.2 | 80 °C/2 h | 0.2477 | 2.8 | 4.9 | 9.3 | 108 | ±11 |
| H2O | H2O | 80 °C/2 h | 0.1976 | 3.5 | 6.1 | 11.7 | 80 | ±11 |
| MS-U | MS untreated | - | 0.2543 | 2.7 | 4.7 | 9.1 | 303 | ±37 |
| 50/50 | MS/RM 50%/50% | - | 0.2428 | 2.9 | 5.0 | 9.5 | 210 | ±16 |
| 75/25 | MS/RM 75%/25% | - | 0.2628 | 2.6 | 4.6 | 8.8 | 255 | ±31 |

2.3. BMP of Rügen-Mix Co-Digested with Maize Silage

2.4. Evaluation of Methane Production Dynamics
2.5. Continuous Reactor Studies: Mesophilic AD

| Mode | Phase | Time [days] | CH4 production [mL·g−1·d−1 VS] | OLR [g·L−1·d−1] | HRT [d] |
|---|---|---|---|---|---|
| Meso | P1 | 4–21 | 73 | 1.0 | 40 |
| P2 | 22–35 | 68 | 1.5 | 40 | |
| P3 | 36–69 | 62 | 2.0 | 40 | |
| P4 | 70–132 | 54 | 2.5 | 40 | |
| P5 | 133–175 | 53 | 2.5 | 31 | |
| Thermo | 4–57 | 65 | 3.0 | 15 |
2.6. Continuous Reactor Studies: Thermophilic AD

2.7. Analysis of Fermentation Residue
| Category | Element | RM | RMR | Digestate | DüMV | Unit |
|---|---|---|---|---|---|---|
| Heavy metals (BioAbfV) | Lead (Pb) | 8.6 | 11 | 2.9 | 150 | mg·kg−1 TS |
| Cadmium (Cd) | 3.2 | 3.8 | 0.26 | 1.5 | ||
| Chromium (Cr) | 13 | 21 | 9.0 | 300 | ||
| Copper (Cu) | 20 | 28 | 69 | 70 | ||
| Nickel (Ni) | 15 | 20 | 7.5 | 80 | ||
| Mercury (Hg) | 0.05 | 0.04 | 0.03 | 1 | ||
| Zinc (Zn) | 141 | 215 | 316 | 500 | ||
| Macronutrients | Phosphorous (P) | 1900 | 2800 | 25,700 | 300 | |
| Potassium (K) | 11,400 | 14,800 | 71,400 | 500 | ||
| Magnesium (Mg) | 6030 | 8120 | 12,000 | 300 | ||
| Calcium (Ca) | 16,200 | 21,300 | 30,000 | 500 | ||
| Sulfur (S) | 19,800 | 21,300 | 4710 | 300 | ||
| C/N ratio | 8.75:1 | 10:1 | 6.4:1 | |||
| Total carbon | 35 | 42 | 43 | % TS | ||
| Total nitrogen | 40,000 | 21,300 | 67,140 | 1000 | mg·kg−1 TS | |
| Micronutrients | Molybdenum (Mo) | 1.2 | 1.6 | 2 | mg·kg−1 TS | |
| Iron (Fe) | 6200 | 8560 | 100 | |||
| Cobalt (Co) | 1.2 | 2.9 | 4 | |||
| Selenium (Se) | 0.8 | 1 | ||||
| Manganese (Mn) | 180 | 280 | 200 |
3. Discussion
3.1. Biomass Composition and Theoretical BMP
3.2. Effect of Acid Hydrolysis on BMP
3.3. Effect of Co-Digestion of Rügen-Mix with Maize Silage on Total BMP
3.4. Effect of Acid Hydrolysis and Co-Digestion on Methane Formation Dynamics
3.5. Biomethane Production from Rügen-Mix during Mesophilic and Thermophilic Continuous Anaerobic Digestion
3.6. Analysis of Fermentation Residue
4. Experimental Section
4.1. Macroalgae Biomass Rügen-Mix and Maize Silage
4.2. Inoculum Sludge
4.3. Flue Gas Condensate
4.4. Acid Hydrolysis Pretreatment
| Name | Reaction time | Temperature | Medium | Concentration |
|---|---|---|---|---|
| RM-U; U | - | - | H2O | - |
| 0.05 M | 2 h | 80 °C | HCl | 0.05 M |
| 0.1 M | 2 h | 80 °C | HCl | 0.1 M |
| 0.2 M | 2 h | 80 °C | HCl | 0.2 M |
| 30 min | 30 min | 80 °C | HCl | 0.2 M |
| 60 min | 60 min | 80 °C | HCl | 0.2 M |
| 90 min | 90 min | 80 °C | HCl | 0.2 M |
| HCl | 2 h | 80 °C | HCl | pH 1.2 |
| FGC | 2 h | 80 °C | FGC | pH 1.2 |
| H2O | 2 h | 80 °C | H2O | - |
| 100 | 2 h | 100 °C | HCl | 0.2 M |
4.5. Biomethane Potential Tests and Batch Array
4.6. Setup Continuous Stirred Tank Reactor (CSTR) 2 L
4.7. Analytical Methods and Calculations
4.7.1. Measurement of Volatile Solids and Total Solids
4.7.2. Data Treatment from Methane Production
4.7.3. Calculations for Comparison of Methane Formation Dynamics in Batch
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Biofuels Sources, Biofuel Policy, Biofuel Economy and Global Biofuel Projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent Trends in Global Production and Utilization of Bio-Ethanol Fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Diekmann, J.; Kemfert, C. Erneuerbare Energien: Weitere Förderung Aus Klimaschutzgründen Unverzichtbar. DIW Wochenber. 2005, 72, 439–449. [Google Scholar]
- Julien, F.; Lamla, M. Competitiveness of Renewable Energies—Comparison of Major European Countries; Discussion Paper No. 302; Department of Business Administration and Economics, European University Viadrina: Frankfurt, Germany, 2011. [Google Scholar]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Lim, S.; Kim, Y.; Park, J.M. Potentials of Macroalgae as Feedstocks for Biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Scharlemann, J.P.W.; Laurance, W.F. How Green are Biofuels? Science 2008, 319, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How Sustainable Agriculture can Address the Environmental and Human Health Harms of Industrial Agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Maeve, S.K.; Black, K.D.; Stanley, M.S. Biogas from Macroalgae: Is it Time to Revisit the Idea? Biotechnol. Biofuels 2012, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zieliński, M.; Grala, A.; Dudek, M. Algae Biomass as an Alternative Substrate in Biogas Production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases through Emissions from Land-use Change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Jin, Y. Marine Macroalgae: An Untapped Resource for Producing Fuels and Chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production—A Feasibility Study with Recommendations for Further Research; Marine Estate Research Report; The Crown Estate: London, UK, 2008; p. 113. [Google Scholar]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas Production from the Brown Seaweed Saccharina Latissima: Thermal Pretreatment and Codigestion with Wheat Straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrère, H. Effect of Thermochemical Pretreatment on the Solubilization and Anaerobic Biodegradability of the Red Macroalga Palmaria Palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Tedesco, S.; Marrero Barroso, T.; Olabi, A.G. Optimization of Mechanical Pre-Treatment of Laminariaceae Spp. Biomass-Derived Biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The Potential of Algae Blooms to Produce Renewable Gaseous Fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Barbot, Y.N.; Falk, H.M.; Benz, R. Thermo-Acidic Pretreatment of Marine Brown Algae Fucus Vesiculosus to Increase Methane Production—A Disposal Principle for Macroalgae Waste from Beaches. J. Appl. Phycol. 2014, 1–9. [Google Scholar] [CrossRef]
- Jung, K.; Kim, D.; Shin, H. Fermentative Hydrogen Production from Laminaria Japonica and Optimization of Thermal Pretreatment Conditions. Bioresour. Technol. 2011, 102, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, D.; Kim, H.; Shin, H. Optimization of Combined (Acid + thermal) Pretreatment for Fermentative Hydrogen Production from Laminaria Japonica using Response Surface Methodology (RSM). Int. J. Hydrog. Energy 2011, 36, 9626–9631. [Google Scholar] [CrossRef]
- Jang, S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Production of Mono Sugar from Acid Hydrolysis of Seaweed. Afr. J. Biotechnol. 2012, 11, 1953–1963. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B.; Bruhn, A. Influence of Variable Feeding on Mesophilic and Thermophilic Co-Digestion of Laminaria Digitata and Cattle Manure. Energy Convers. Manag. 2014, 87, 513–520. [Google Scholar] [CrossRef]
- Murphy, J.D.; Thamsiriroj, T. Fundamental science and engineering of the anaerobic digestion process. In Biogas Handbook: Science, Production and Applications; Wellinger, A., Murphy, J.D., Baxter, D., Eds.; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation Potential of Macroalgae Ulva Spp. and Gracilaria Spp. and in Co-Digestion with Waste Activated Sludge. Bioresour. Technol. 2012, 114, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany Macroalgae Screening: Composition and Methane Potential for Potential Alternative Sources of Energy and Products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Migliore, G.; Alisi, C.; Sprocati, A.R.; Massi, E.; Ciccoli, R.; Lenzi, M.; Wang, A.; Cremisini, C. Anaerobic Digestion of Macroalgal Biomass and Sediments Sourced from the Orbetello Lagoon, Italy. Biomass Bioenergy 2012, 42, 69–77. [Google Scholar] [CrossRef]
- Matsui, T.; Koike, Y. Methane Fermentation of a Mixture of Seaweed and Milk at a Pilot-Scale Plant. J. Biosci. Bioeng. 2010, 110, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, G.; Lubecki, L.; Szymczak-Żyła, M.; Bucholc, K.; Filipkowska, A.; Gogacz, R.; Zamojska, A. Eutrophication Monitoring System Near the Sopot Beach (Southern Baltic). Ocean Coast. Manag. 2014, 98, 51–61. [Google Scholar] [CrossRef]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal Blooms in Shallow Estuaries: Controls and Ecophysiological and Ecosystem Consequences. Am. Soc. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Briand, X.; Morand, P. Anaerobic Digestion of Ulva Sp. 1. Relationship between Ulva Composition and Methanisation. J. Appl. Phycol. 1997, 9, 511–524. [Google Scholar] [CrossRef]
- Bucholc, K.; Szymczak-Żyła, M.; Lubecki, L.; Zamojska, A.; Hapter, P.; Tjernström, E.; Kowalewska, G. Nutrient Content in Macrophyta Collected from Southern Baltic Sea Beaches in Relation to Eutrophication and Biogas Production. Sci. Total Environ. 2014, 473–474, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Mossbauer, M.; Haller, I.; Dahlke, S.; Schernewski, G. Management of Stranded Eelgrass and Macroalgae Along the German Baltic Coastline. Ocean Coast. Manag. 2012, 57, 1–9. [Google Scholar] [CrossRef]
- Charlier, R.H.; Morand, P.; Finkl, C.W.; Thys, A. Green Tides on the Brittany Coasts. Environ. Res. Eng. Manag. 2007, 3, 52–59. [Google Scholar] [CrossRef]
- Charlier, R.H.; Lonhienne, T. The Management of Eutrophicated Waters. In Marine Benthic Vegetation; Schramm, W., Nienhuis, P., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 1996; Volume 123, pp. 45–78. [Google Scholar]
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, T.; Meng, G.; Wu, L.; Zhang, X.; Guo, W. Numerical Simulation for Effects of Hydrodynamic Condition on Algae Growth in Chongqing Reaches of Jialing River. Ecol. Model. 2011, 222, 112–119. [Google Scholar] [CrossRef]
- Filipkowska, A.; Lubecki, L.; Szymczak-Żyła, M.; Łotocka, M.; Kowalewska, G. Factors Affecting the Occurrence of Algae on the Sopot Beach (Baltic Sea). Oceanologia 2009, 51, 233–262. [Google Scholar] [CrossRef]
- Aderhold, D.; Williams, C.J.; Edyvean, R.G.J. The Removal of Heavy-Metal Ions by Seaweeds and their Derivatives. Bioresour. Technol. 1996, 58, 1–6. [Google Scholar] [CrossRef]
- Felgentreu, C. Mais Unter Trockenen Bedingungen Produzieren—Düngungsstrategien Bei Wassermangel (Teil 2). Innovation 2007, 1, 14–17. [Google Scholar]
- FNR. Biogas Broschüre; FNR: Gülzow-Prüzen, Germany, 2013; p. 44. [Google Scholar]
- LfU. Biogashandbuch Bayern. 2007. Available online: http://www.lfu.bayern.de/energie/biogashandbuch/doc/kap1bis15.pdf (accessed on 21 May 2015).
- VDI-4630. Fermentation of Organic Materials—Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI: Duesseldorf, Germany, 2006; p. 92. [Google Scholar]
- FNR. Guide to Biogas—From Production to Use. 2012. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/g/u/guide_biogas_engl_2012.pdf (accessed on 21 May 2015).
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources—An Introduction; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 443. [Google Scholar]
- Demirel, B.; Scherer, P. Trace Element Requirements of Agricultural Biogas Digesters during Biological Conversion of Renewable Biomass to Methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Design of Experiments to Assess Pre-Treatment and Co-Digestion Strategies that Optimize Biogas Production from Macroalgae Gracilaria Vermiculophylla. Bioresour. Technol. 2014, 162, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babujanarthanam, R.; Kavitha, P. Simultaneous Saccharification and Fermentation of Dilute Acid Pretreated Red Algae (Gelidiella Acerosa) for Bioethanol Production. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 1305–1314. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the Macroalgae Chaetomorpha Linum for the Production of Bioethanol—Comparison of Five Pretreatment Technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. Investigation of the Optimal Percentage of Green Seaweed that may be Co-Digested with Dairy Slurry to Produce Gaseous Biofuel. Bioresour. Technol. 2014, 170, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, L.F.R.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production; IEA Bioenergy: Paris, France, 2014; p. 24. [Google Scholar]
- Hinks, J.; Edwards, S.; Sallis, P.J.; Caldwell, G.S. The Steady State Anaerobic Digestion of Laminaria Hyperborea—Effect of Hydraulic Residence on Biogas Production and Bacterial Community Composition. Bioresour. Technol. 2013, 143, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Habig, C.; Ryther, J.H. Methane Production from the Anaerobic Digestion of some Marine Macrophytes. Resour. Conserv. 1983, 8, 271–279. [Google Scholar] [CrossRef]
- Biswas, R. Biomethanation of Red Algae from the Eutrophied Baltic Sea. Master’s Thesis, Linköping University, Linköping, Sweden, 2009. [Google Scholar]
- Scherer, P. Bestimmung Der Abbauraten Von Biogasanlagen. 2006. Available online: http://www.eti-brandenburg.de/fileadmin/eti_upload/downloads2008/06_Scherer.pdf (accessed on 21 May 2015).
- Nielsen, H.B.; Heiske, S. Anaerobic Digestion of Macroalgae: Methane Potentials, Pre-Treatment, Inhibition and Co-Digestion. Water Sci. Technol. 2011, 64, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; König, R. From a Black-Box to a Glass-Box System: The Attempt Towards a Plant-Wide Automation Concept for Full-Scale Biogas Plants. Water Sci. Technol. 2009, 60, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G. Methane Production from Marine, Green Macro-Algae. Resour. Conserv. 1983, 8, 185–194. [Google Scholar] [CrossRef]
- Bergström, K. Impact on using Macroalgae from the Baltic Sea in Biogas Production—A Review with Special Emphasis on Heavy Metals. Master’s Thesis, Linnæus University, Kalmar, Sweden, 2012. [Google Scholar]
- Stirk, W.A.; van Staden, J. Removal of Heavy Metals from Solution using Dried Brown Seaweed Material. Bot. Mar. 2000, 43, 467–473. [Google Scholar] [CrossRef]
- Filipkowska, A.; Lubecki, L.; Szymczak-Żyła, M.; Kowalewska, G.; Żbikowski, R.; Szefer, P. Utilisation of Macroalgae from the Sopot Beach (Baltic Sea). Oceanologia 2008, 50, 255–273. [Google Scholar]
- Nkemka, V.N.; Murto, M. Exploring Strategies for Seaweed Hydrolysis: Effect on Methane Potential and Heavy Metal Mobilisation. Process Biochem. 2012, 47, 2523–2526. [Google Scholar] [CrossRef]
- FNR. Bioenergy in Germany—Facts and Figures. 2013. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/b/a/basisdaten_9x16_2013_engl_web.pdf (accessed on 9 August 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbot, Y.N.; Thomsen, L.; Benz, R. Thermo-Acidic Pretreatment of Beach Macroalgae from Rügen to Optimize Biomethane Production—Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management. Mar. Drugs 2015, 13, 5681-5705. https://doi.org/10.3390/md13095681
Barbot YN, Thomsen L, Benz R. Thermo-Acidic Pretreatment of Beach Macroalgae from Rügen to Optimize Biomethane Production—Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management. Marine Drugs. 2015; 13(9):5681-5705. https://doi.org/10.3390/md13095681
Chicago/Turabian StyleBarbot, Yann Nicolas, Laurenz Thomsen, and Roland Benz. 2015. "Thermo-Acidic Pretreatment of Beach Macroalgae from Rügen to Optimize Biomethane Production—Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management" Marine Drugs 13, no. 9: 5681-5705. https://doi.org/10.3390/md13095681