In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Pardaxin-Induced Inhibition of Cell Growth in SCC-4 Cells


2.2. Pardaxin-Induced Apoptosis in OSCC Cells


2.3. Cell Cycle of OSCC Cells after Treatment with Pardaxin

| G0/G1 | S | G2/M | |
|---|---|---|---|
| Untreated | 73.6 ± 5.22 a | 6.6 ± 0.15 b | 19.8 ± 2.05 c |
| 5 μg/mL | 67.7 ± 4.98 a | 5.9 ± 0.14 b | 26.4 ± 1.73 b |
| 10 μg/mL | 59.0 ± 4.96 b | 10.2 ± 0.36 a | 30.8 ± 3.91 b |
| 25 μg/mL | 54.4 ± 4.11 b | 8.6 ± 0.08 a | 37.0 ± 3.24 a |

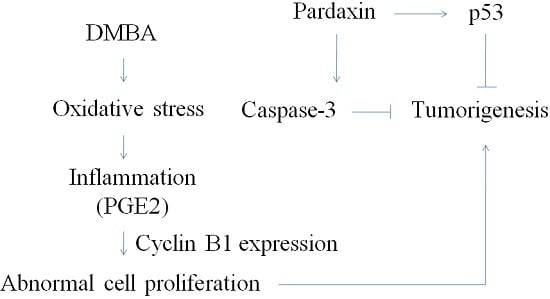
2.4. Pardaxin-Induced Reduction in Carcinogenesis in DMBA-Induced Hamster Buccal Pouch Model

| Group | Mean Tumor Volume (mm3) | Tumor Burden (mm3) | Mean Tumor Number/Hamster |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| DMBA | 131.4 | 417.6 | 13.5 |
| DMBA + Pardaxin (25 mg/kg bw) | 138.9 | 398.1 | 12.9 |
| DMBA + Pardaxin (50 mg/kg bw) | 89.3 | 237.7 | 9.1 |
| DMBA + Pardaxin (75 mg/kg bw) | 36.2 | 74.8 | 5.1 |
| DMBA + 5-FU (25 mg/kg bw) | 41.5 | 95.4 | 4.8 |
| Pardaxin (75 mg/kg bw) | 0 | 0 | 0 |
2.5. Inhibition of PGE2Levels in DMBA-Induced Hamster Buccal Pouch Tumor Model by Pardaxin

3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. MTT Assay
4.3. Colony Formation
4.4. Apoptosis
4.5. Cell Cycle
4.6. Western Blot Technique
4.7. Syrian Hamster Model
4.8. Assay for PGE2 Level
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Protection of Monascus-fermented dioscorea against DMBA-induced oral injury in hamster by anti-inflammatory and antioxidative potentials. J. Agric. Food Chem. 2010, 58, 6715–6720. [Google Scholar] [PubMed]
- Chen, Y.J.; Chang, J.T.; Liao, C.T.; Wang, H.M.; Yen, T.C.; Chiu, C.C.; Lu, Y.C.; Li, H.F.; Cheng, A.J. Head and neck cancer in the betel quid chewing area: Recent advances in molecular carcinogenesis. Cancer Sci. 2008, 99, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Effects of red mold dioscorea on oral carcinogenesis in DMBA-induced hamster animal model. Food Chem. Toxicol. 2011, 49, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Jung, W.K.; Mendis, E.; Moon, S.H.; Kim, S.K. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005, 76, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta. 2014, 1838, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Chen, J.Y.; Wu, C.J. Enhanced control of bladder-associated tumors using shrimp anti-lipopolysaccharide factor (SALF) antimicrobial peptide as a cancer vaccine adjuvant in mice. Mar. Drugs 2015, 13, 3241–3258. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Lee, J.F.; Chen, J.Y. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT1080 cells. Mar. Drugs 2011, 9, 1995–2009. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Ivestig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Lin, C.N.; Chiou, M.T.; Yu, C.Y.; Chen, J.Y.; Chien, C.H. The antimicrobial peptide pardaxin exerts potent anti-tumor activity against canine perianal gland adenoma. Oncotarget 2014, 6, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Wu, C.J.; Chen, J.Y. A cancer vaccine based on the marine antimicrobial peptide pardaxin (GE33) for control of bladder-associated tumors. Biomaterials 2013, 34, 10151–10159. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Huang, T.C.; Lin, C.C.; Hui, C.F.; Lin, C.H.; Chen, J.Y. Pardaxin, a fish antimicrobial peptide, exhibits antitumor activity toward murine fibrosarcoma in vitro and in vivo. Mar. Drugs 2012, 10, 1852–1872. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Takacsi-Nagy, Z.; Hitre, E.; Remenar, E.; Oberna, F.; Polgar, C.; Major, T.; Godeny, M.; Fodor, J.; Kasler, M. Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III–IV unresectable head and neck cancer. Strahlenther. Onkol. 2015, 191, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.H.; Wang, Y.J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.K.; Sun, M. Antitumor peptides from marine organisms. Mar.Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, M.; Hu, C.; Liao, D.J. Targeting cellular proapoptotic molecules for developing anticancer agents from marine sources. Curr. Drug Targets 2010, 11, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Ceron, J.M.; Contreras-Moreno, J.; Puertollano, E.; de Cienfuegos, G.A.; Puertollano, M.A.; de Pablo, M.A. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides 2010, 31, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Pan, C.Y.; Rajanbabu, V.; Cheng, C.W.; Chen, J.Y. Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1–5, shows antitumor activity in cancer cells. Peptides 2011, 32, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Chen, J.Y. Proteomic analysis reveals that pardaxin triggers apoptotic signaling pathways in human cervical carcinoma HeLa cells: Cross talk among the UPR, c-Jun and ROS. Carcinogenesis 2013, 34, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.C.; Chen, J.Y. Pardaxin-indueced apoptosis enhances antitumor activity in HeLa cells. Peptides 2011, 32, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Meek, D.W. Regulation of the p53 response and its relationship to cancer. Biochem. J. 2015, 469, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; DuBois, R.N. COX-2: A target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Siena, L.; Ferraro, M.; Profita, M.; Mondello, P.; Chiappara, G.; Montalbano, A.M.; Giarratano, A.; Bonsignore, G.; Gjomarkaj, M. Role of prostaglandin E2 in the invasiveness, growth and protection of cancer cells in malignant pleuritis. Eur. J. Cancer 2006, 42, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nakayama, H.; Nagata, M.; Yoshida, R.; Kawahara, K.; Hirosue, A.; Tanaka, T.; Yuno, A.; Matsuoka, Y.; Kojima, T.; et al. Overexpression of fibronectin confers cell adhesion-mediated drug resistance (CAM-DR) against 5-FU in oral squamous cell carcinoma cells. Int. J. Oncol. 2014, 44, 1376–1384. [Google Scholar] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013, 19, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Moraitis, D.; Du, B.; de Lorenzo, M.S.; Boyle, J.O.; Weksler, B.B.; Cohen, E.G.; Carew, J.F.; Altorki, N.K.; Kopelovich, L.; Subbaramaiah, K.; et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: Evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005, 65, 664–670. [Google Scholar] [PubMed]
- Cebola, I.; Peinado, M.A. Epigenetic deregulation of the COX pathway in cancer. Prog. Lipid Res. 2012, 51, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Red mold dioscorea-indueced G2/M arrest and apoptosis in human oral cancer cells. J. Sci. Food Agric. 2010, 90, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Cui, Z.; Li, Y.-H.; Hsu, W.-H.; Lee, B.-H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Mar. Drugs 2016, 14, 2. https://doi.org/10.3390/md14010002
Han Y, Cui Z, Li Y-H, Hsu W-H, Lee B-H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Marine Drugs. 2016; 14(1):2. https://doi.org/10.3390/md14010002
Chicago/Turabian StyleHan, Yifan, Zhibin Cui, Yen-Hsing Li, Wei-Hsuan Hsu, and Bao-Hong Lee. 2016. "In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma" Marine Drugs 14, no. 1: 2. https://doi.org/10.3390/md14010002
APA StyleHan, Y., Cui, Z., Li, Y.-H., Hsu, W.-H., & Lee, B.-H. (2016). In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Marine Drugs, 14(1), 2. https://doi.org/10.3390/md14010002