Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease
Abstract
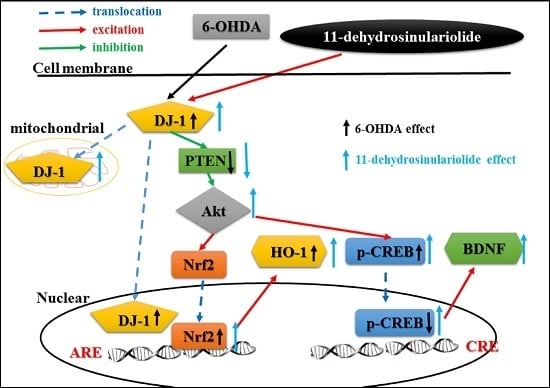
:1. Introduction
2. Results
2.1. 11-de Enhanced 6-OHDA-Induced Upregulation of DJ-1 Expression
2.2. Effect of 11-de on PTEN, Akt, HO-1, and Nrf2 Protein Expression, and Nrf2 Translocation in SH-SY5Y Cells
2.3. Effect of 11-de on 6-OHDA-Induced Upregulation of Phospho-cAMP Response Element-Binding Protein (p-CREB) and Brain-Derived Neurotrophic Factor (BDNF) Expression
2.4. The Effect of 11-de on p-Akt, HO-1, SOD-1, and Neuroprotective Activity in DJ-1-Knockdown Cells
2.5. The Protective Effects of 11-de on Locomotor Activity and Protein Expression in 6-OHDA-Treated Zebrafish
2.6. Pretreatment with 11-de for 6-OHDA-Induced Lesions in a Rat Model of PD
3. Discussion
3.1. Summary
3.2. Functions of DJ-1 in Different Locations
3.3. Effect of Neuroprotective Agents on Nrf2/HO-1 and CREB/BDNF Pathways
3.4. Future Studies Involving 11-Dehydrosinulariolide
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Nuclear Extracts
4.3. Electrophoretic Mobility Shift Assay (EMSA)
4.4. Preparation of Mitochondrial Extracts
4.5. Western Blotting
4.6. Quantitative Real-Time PCR
4.7. Transfection of DJ-1 siRNA
4.8. Zebrafish and Locomotor Test
4.9. Ethical Approval
4.10. Animals
4.11. 6-OHDA-Induced Parkinson’s Study in Rats
4.12. Immunohistochemistry
4.13. Statistical Analysis
4.14. Chemical and Antibodies
- 6-OHDA (6-hydroxydopamine, Sigma, St. Louis, MO, USA; catalog No. H4381)
- β-actin (a loading control; dilution, 1:1000) (Sigma, St. Louis, MO, USA; catalog No. A5441)
- BDNF (brain derived neurotrophic factor, dilution 1:100) (Millipore, Billerica, MA, USA; catalog No. AB1779SP)
- HO-1 (heme oxygenase-1, dilution, 1:1000) (Cell Signaling Technology, Danvers, MA, USA; catalog No. 5061)
- Nrf2 (dilution, 1:1000) (Abcam, biorbyt, Cambridge, UK; catalog No. ab31163)
- PARK7/DJ-1 (dilution, 1:1000) (Abcam, biorbyt, Cambridge, UK; catalog No. ab18257)
- p-Akt (Protein kinase B; dilution, 1:1000) (Cell Signaling Technology, Danvers, MA, USA; catalog No. 9271)
- p-CREB (cAMP response element binding protein, dilution, 1:1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA; catalog No. sc-7978)
- TH (tyrosine hydroxylase; dilution 1:1000) (Millipore, Billerica, MA, USA; catalog No. MAB318)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thanvi, B.R.; Lo, T.C. Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E. Early-morning dystonia. A late side effect of long-term levodopa therapy in Parkinson’s disease. Arch. Neurol. 1979, 36, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Markham, C.H.; Diamond, S.G. Long-term follow-up of early dopa treatment in Parkinson’s disease. Ann. Neurol. 1986, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Herin, M.; Colin, M.; Tursch, B. Chemical Studies of Marine-Invertebrates. 25. Bull. Soc. Chim. Belg. 1976, 85, 801–803. [Google Scholar]
- Lin, Y.S.; Chen, C.H.; Liaw, C.C.; Chen, Y.C.; Kuo, Y.H.; Shen, Y.C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Liu, C.I.; Chen, C.C.; Chen, J.C.; Su, J.H.; Huang, H.H.; Chen, J.Y.; Wu, Y.J. Proteomic analysis of anti-tumor effects of 11-dehydrosinulariolide on CAL-27 cells. Mar. Drugs 2011, 9, 1254–1272. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Tsai, F.J.; Lin, J.J.; Huang, H.H.; Chiu, C.C.; Su, J.H.; Yang, Y.T.; Chen, J.Y.; Wong, B.S.; Wu, Y.J. Induction of apoptosis by 11-dehydrosinulariolide via mitochondrial dysregulation and ER stress pathways in human melanoma cells. Mar. Drugs 2012, 10, 1883–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Chakraborty, C.; Sung, C.S.; Feng, C.W.; Jean, Y.H.; Lin, Y.Y.; Hung, H.C.; Huang, T.Y.; Huang, S.Y.; Su, T.M.; et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s model: A promising candidate for the treatment of Parkinson’s disease. Naunyn-Schmiedebergs Arch. Pharmacol. 2012, 385, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sleiman, P.M.; Healy, D.G.; Quinn, N.; Lees, A.J.; Wood, N.W. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann. Neurol. 2003, 54, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.G.; Abou-Sleiman, P.M.; Valente, E.M.; Gilks, W.P.; Bhatia, K.; Quinn, N.; Lees, A.J.; Wood, N.W. DJ-1 mutations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 144–145. [Google Scholar] [PubMed]
- Van Duijn, C.M.; Dekker, M.C.; Bonifati, V.; Galjaard, R.J.; Houwing-Duistermaat, J.J.; Snijders, P.J.; Testers, L.; Breedveld, G.J.; Horstink, M.; Sandkuijl, L.A.; et al. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am. J. Hum. Genet. 2001, 69, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Taira, T.; Kitamura, Y.; Yanagida, T.; Tsuchiya, D.; Takata, K.; Yanagisawa, D.; Nishimura, K.; Taniguchi, T.; Kiso, Y.; et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol. Dis. 2006, 24, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, W.; Qi, Z.; Lu, L.; Duan, C.; Zhao, C.; Yang, H. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J. Mol. Biol. 2012, 423, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475–12480. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.C.; Bohmann, D. Assessing neurodegenerative phenotypes in Drosophila dopaminergic neurons by climbing assays and whole brain immunostaining. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Mylonakou, M.N.; Skare, O.; Amiry-Moghaddam, M.; Torp, R. Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res. 2011, 1378, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.W.; Wen, Z.H.; Huang, S.Y.; Hung, H.C.; Chen, C.H.; Yang, S.N.; Chen, N.F.; Wang, H.M.; Hsiao, C.D.; Chen, W.F. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shimoji, M.; Thomas, B.; Moore, D.J.; Yu, S.W.; Marupudi, N.I.; Torp, R.; Torgner, I.A.; Ottersen, O.P.; Dawson, T.M.; et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: Implications for pathogenesis. Hum. Mol. Genet. 2005, 14, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Lee, K.W.; Junn, E.; Mouradian, M.M. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci. Res. 2010, 67, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, Y.J.; Hwang, I.Y.; Youdim, M.B.; Park, K.S.; Oh, Y.J. Nuclear translocation of DJ-1 during oxidative stress-induced neuronal cell death. Free Radic. Biol. Med. 2012, 53, 936–950. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, D.J.; Jeong, H.K.; Kim, J.; Kim, D.W.; Choi, S.Y.; Park, S.M.; Suh, Y.H.; Jou, I.; Joe, E.H. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 2013, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Birkmayer, W.; Riederer, P.; Ambrozi, L.; Youdim, M.B. Implications of combined treatment with ‘Madopar’ and l-deprenil in Parkinson’s disease. A long-term study. Lancet 1977, 1, 439–443. [Google Scholar] [CrossRef]
- Xiao, H.; Lv, F.; Xu, W.; Zhang, L.; Jing, P.; Cao, X. Deprenyl prevents MPP+-induced oxidative damage in PC12 cells by the upregulation of Nrf2-mediated NQO1 expression through the activation of PI3K/Akt and Erk. Toxicology 2011, 290, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Nakaso, K.; Nakamura, C.; Sato, H.; Imamura, K.; Takeshima, T.; Nakashima, K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem. Biophys. Res. Commun. 2006, 339, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.M.; Mohanakumar, K.P.; Murphy, D.L.; Chiueh, C.C. Antioxidant mechanism and protection of nigral neurons against MPP+ toxicity by deprenyl (selegiline). Ann. N. Y. Acad. Sci. 1994, 738, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Tipton, K.F. Rat striatal monoamine oxidase-B inhibition by l-deprenyl and rasagiline: Its relationship to 2-phenylethylamine-induced stereotypy and Parkinson’s disease. Parkinsonism Relat. Disord. 2002, 8, 247–253. [Google Scholar] [CrossRef]
- Schroeter, H.; Bahia, P.; Spencer, J.P.; Sheppard, O.; Rattray, M.; Cadenas, E.; Rice-Evans, C.; Williams, R.J. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J. Neurochem. 2007, 101, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.S.; Ying, Z.; Zhuang, Y.; Zhong, H.; Wu, A.; Bhatia, H.S.; Cruz, R.; Tillakaratne, N.J.; Roy, R.R.; Edgerton, V.R.; et al. Effects of diet and/or exercise in enhancing spinal cord sensorimotor learning. PLoS ONE 2012, 7, e41288. [Google Scholar] [CrossRef] [PubMed]
- Jagatha, B.; Mythri, R.B.; Vali, S.; Bharath, M.M. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: Therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic. Biol. Med. 2008, 44, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Vilotti, S.; Codrich, M.; Dal Ferro, M.; Pinto, M.; Ferrer, I.; Collavin, L.; Gustincich, S.; Zucchelli, S. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS ONE 2012, 7, e35051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Sung, C.S.; Duh, C.Y.; Huang, S.Y.; Lin, C.S.; Tai, M.H.; Tzeng, S.F.; Wen, Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory. Animals Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE, etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol. Scand. 1971, 82 (Suppl. S367), 49–68. [Google Scholar] [CrossRef]
- Pycock, C.J. Turning behaviour in animals. Neuroscience 1980, 5, 461–514. [Google Scholar] [CrossRef]
- Carman, L.S.; Gage, F.H.; Shults, C.W. Partial lesion of the substantia nigra: Relation between extent of lesion and rotational behavior. Brain Res. 1991, 553, 275–283. [Google Scholar] [CrossRef]
- Allbutt, H.N.; Henderson, J.M. Use of the narrow beam test in the rat, 6-hydroxydopamine model of Parkinson’s disease. J. Neurosci. Methods 2007, 159, 195–202. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.-W.; Hung, H.-C.; Huang, S.-Y.; Chen, C.-H.; Chen, Y.-R.; Chen, C.-Y.; Yang, S.-N.; Wang, H.-M.D.; Sung, P.-J.; Sheu, J.-H.; et al. Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease. Mar. Drugs 2016, 14, 187. https://doi.org/10.3390/md14100187
Feng C-W, Hung H-C, Huang S-Y, Chen C-H, Chen Y-R, Chen C-Y, Yang S-N, Wang H-MD, Sung P-J, Sheu J-H, et al. Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease. Marine Drugs. 2016; 14(10):187. https://doi.org/10.3390/md14100187
Chicago/Turabian StyleFeng, Chien-Wei, Han-Chun Hung, Shi-Ying Huang, Chun-Hong Chen, Yun-Ru Chen, Chun-Yu Chen, San-Nan Yang, Hui-Min David Wang, Ping-Jyun Sung, Jyh-Horng Sheu, and et al. 2016. "Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease" Marine Drugs 14, no. 10: 187. https://doi.org/10.3390/md14100187
APA StyleFeng, C. -W., Hung, H. -C., Huang, S. -Y., Chen, C. -H., Chen, Y. -R., Chen, C. -Y., Yang, S. -N., Wang, H. -M. D., Sung, P. -J., Sheu, J. -H., Tsui, K. -H., Chen, W. -F., & Wen, Z. -H. (2016). Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease. Marine Drugs, 14(10), 187. https://doi.org/10.3390/md14100187