Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo
Abstract
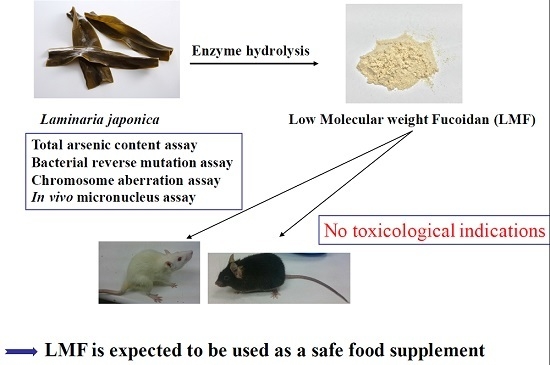
:1. Introduction
2. Results and Discussion
2.1. Total Arsenic and Inorganic Arsenic Content of Laminaria japonica and LMF-LJ
2.2. Bacterial Reverse Mutation Assay
2.3. Chromosome Aberration Assay
3. Materials and Methods
3.1. Low Molecular Weight Fucoidan
3.2. Determination of Total Arsenic and Inorganic Arsenic Species
3.3. Bacterial Reverse Mutation Assay
3.4. Chromosome Aberration Assay
3.5. In Vivo Mouse Micronucleus Assay
3.6. In Vivo Rat Repeated-Dose 28-Day Oral Toxicity Assay
3.6.1. Body Weight, Food Intake, and Water Consumption
3.6.2. Observation of Clinical Signs
3.6.3. Urinalysis
3.6.4. Hematology
3.6.5. Serum Biochemistry
3.6.6. Organ Weight
3.6.7. Histopathology
3.7. Statistical Analysis
4. In Vivo Mouse Micronucleus Assay
5. In Vivo Rat Repeated Dose 28-Day Oral Toxicity Assay
5.1. Body Weights, Food Intake, Water Consumption, and Clinical Signs
5.2. Urinalysis Results
5.3. Hematological, Blood Clotting, and Serum Biochemistry Results
5.4. Organ Weight and Histopathological Results
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lincoln, R.A.; Strupinski, K.; Walker, J.M. Bioactive compounds from algae. Life Chem. Rep. 1991, 8, 97–183. [Google Scholar]
- Kylin, H. Zur biochemie der meeresalgen. HoppeSeylers Z. Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low molecular weight fucoidan inhibits tumor angiogenesis through downregulation of HIF-1/VEGF signaling under hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Lin, T.-Y.; Wu, Y.-C.; Tsao, S.-M.; Hwang, P.-A.; Shih, Y.-W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFβ receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed]
- Tengdelius, M.; Lee, C.-J.; Grenegård, M.; Griffith, M.; Påhlsson, P.; Konradsson, P. Synthesis and biological evaluation of fucoidan-mimetic glycopolymers through cyanoxyl-mediated free-radical polymerization. Biomacromolecules 2014, 15, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-A.; Yi, B.-R.; Choi, K.-C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Hung, Y.-L.; Chien, S.-Y. Inhibitory activity of sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J. Food Drug Anal. 2015, 23, 49–56. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul. Fibrinolysis 2009, 20, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Hung, Y.-L.; Phan, N.N.; Hieu, B.-T.-N.; Chang, P.-M.; Li, K.-L.; Lin, Y.-C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chung, D.; Shin, I.-S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Soeda, S.; Sakaguchi, S.; Shimeno, H.; Nagamatsu, A. Fibrinolytic and anticoagulant activities of highly sulfated fucoidan. Biochem. Pharmacol. 1992, 43, 1853–1858. [Google Scholar] [CrossRef]
- Park, S.-B.; Chun, K.-R.; Kim, J.-K.; Suk, K.; Jung, Y.-M.; Lee, W.-H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. PTR 2010, 24, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, Z.-G.; Liu, H.; Wu, C. A new method of laminaria japonica strain selection and sporeling raising by the use of gametophyte clones. Hydrobiologia 1999, 398, 473–476. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.; Jeon, Y.J.P. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Velez, D.; Montoro, R.; Barbera, R.; Farré, R. Estimation of arsenic bioaccessibility in edible seaweed by an in vitro digestion method. J. Agric. Food Chem. 2003, 51, 6080–6085. [Google Scholar] [CrossRef] [PubMed]
- Dopp, E.; Hartmann, L.; Florea, A.-M.; von Recklinghausen, U.; Pieper, R.; Shokouhi, B.; Rettenmeier, A.; Hirner, A.; Obe, G. Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in chinese hamster ovary (cho) cells. Toxicol. Appl. Pharmacol. 2004, 201, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cao, X.; Yu, J.-J.; Wang, X.-R.; Shen, Y. Arsenic speciation in sargassum fusiforme by microwave-assisted extraction and LC-ICP-MS. Chromatographia 2009, 69, 587–591. [Google Scholar] [CrossRef]
- Yamamoto, S.; Konishi, Y.; Matsuda, T.; Murai, T.; Shibata, M.-A.; Matsui-Yuasa, I.; Otani, S.; Kuroda, K.; Endo, G.; Fukushima, S. Cancer induction by an organic arsenic compound, dimethylarsinic acid (cacodylic acid), in F344/DuCrj rats after pretreatment with five carcinogens. Cancer Res. 1995, 55, 1271–1276. [Google Scholar] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wang, C.-J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990, 50, 5470–5474. [Google Scholar] [PubMed]
- Bates, M.N.; Smith, A.H.; Hopenhayn-Rich, C. Arsenic ingestion and internal cancers: A review. Am. J. Epidemiol. 1992, 135, 462–476. [Google Scholar] [PubMed]
- Endo, G.; Kuroda, K.; Okamoto, A.; Horiguchi, S.I. Dimethylarsenic acid induces tetraploids in Chinese hamster cells. Bull. Environ. Contam. Toxicol. 1992, 48, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, D.; Ning, J.; Zhai, Y. Arsenic and cadmium in the marine macroalgae (porphyra yezoensis and Laminaria japonica)—Forms and concentrations. Chem. Speciat. Bioavailab. 2012, 24, 197–203. [Google Scholar] [CrossRef]
- Taiwan's “Act Governing Food Sanitation”, Algae food hygiene standards issue; Taipei, Taiwan, 2013.
- Sha, J.H.H.C.X.; Li, S.Q.; Ni, M.Z.; Deng, X.Y.; Cai, Y.P.; Yuan, B.J. Hygienic Standard for Marine Algae and Algae Products; Standardization Administration of China: Beijing, China, 2005. [Google Scholar]
- Food Standards Australia New Zealand. In Food Standards Code; Australian Government Publishing Service: Kingston, Australia, 1994.
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, O.-H.; Lee, B.-Y. Genotoxicity studies on fucoidan from sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Han, J.S. Genotoxicity testing of low molecular weight fucoidan from brown seaweeds. Food Chem. Toxicol. 2012, 50, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Claxton, L.D.; Allen, J.; Auletta, A.; Mortelmans, K.; Nestmann, E.; Zeiger, E. Guide for the Salmonella typhimurium/mammalian microsome tests for bacterial mutagenicity. Mutat. Res./Genet. Toxicol. 1987, 189, 83–91. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation Development (OECD). Test No. 471: Bacterial Reverse Mutation Test; Organisation for Economic Co-operation Development Publishing: Paris, France, 1997. [Google Scholar]
- Galloway, S.; Armstrong, M.; Reuben, C.; Colman, S.; Brown, B.; Cannon, C.; Bloom, A.; Nakamura, F.; Ahmed, M.; Duk, S. Chromosome aberrations and sister chromatid exchanges in chinese hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol. Mutagen. 1987, 10, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S. International workshop on standardisation of genotoxicity test procedures. Commentary. Mutat. Res. 1994, 312, 201. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, O.-H.; Lee, H.-H.; Lee, B.-Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of Undaria pinnatifida in sprague-dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation Development (OECD). 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; Organisation for Economic Co-operation Development Guidelines For the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2008. [Google Scholar]
- Chung, H.J.; Jeun, J.; Houng, S.J.; Jun, H.J.; Kweon, D.K.; Lee, S.J. Toxicological evaluation of fucoidan from Undaria pinnatifidain vitro and in vivo. Phytother. Res. 2010, 24, 1078–1083. [Google Scholar] [PubMed]
- Organisation for Economic Co-operation Development (OECD). 423: Acute Oral Toxicity-Acute Toxic Class Method; Organisation for Economic Co-operation Development Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Gideon, T.P.; Rengasamy, R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J. Med. Food 2008, 11, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Zhao, T.; Qi, H.; Xu, Z.; Li, Z. Fucoidan inhibits the development of proteinuria in active heymann nephritis. Phytother. Res. 2005, 19, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, K.; Srivastava, R.; Fathzadeh, M.; Li, N.; Mani, A. The protective effect of transcription factor 7-like 2 risk allele rs7903146 against elevated fasting plasma triglyceride in type 2 diabetes: A meta-analysis. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.; Rajendran, M.; Pasanban, B.; Rengasamy, R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine 2010, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Villano, J.S. The Laboratory Rat; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Abe, S.; Hiramatsu, K.; Ichikawa, O.; Kawamoto, H.; Kasagi, T.; Miki, Y.; Kimura, T.; Ikeda, T. Safety evaluation of excessive ingestion of mozuku fucoidan in human. J. Food Sci. 2013, 78, T648–T651. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nagashima, M.; Ghazizadeh, M.; Kawanami, O. Increased effect of fucoidan on lipoprotein lipase secretion in adipocytes. Life Sci. 2009, 84, 523–529. [Google Scholar] [CrossRef] [PubMed]

| Species | Laminaria japonica (mg/kg) | LMF-LJ (mg/kg) |
|---|---|---|
| AsIII | ND a | ND |
| AsV | ND | ND |
| MMA | 9.27 ± 0.96 | 1.35 ± 0.63 |
| DMA | 9.23 ± 0.83 | ND |
| AsB | 34.31 ± 1.21 | 4.77 ± 0.88 |
| AsC | 6.19 ± 2.17 | ND |
| Total arsenic (sum) | 59.00 ± 1.65 | 6.12 ± 2.14 |
| Total arsenic (direct) | 61.100 ± 3.110 | 6.200 ± 2.005 |
| LMF-LJ (μg/mL) | S9 | Average Number of Revertants (Number of Colonies/Plate) | ||||
|---|---|---|---|---|---|---|
| Frameshift | Base Pair Substitution | Transition | ||||
| TA97a | TA98 | TA100 | TA1535 | TA102 | ||
| Negative control | – | 109.7 ± 7.2 a | 17.3 ± 2.9 | 102.0 ± 9.5 | 11.7 ± 1.5 | 475.0 ± 27.7 |
| 312.5 | – | 111.0 ± 5.5 | 14.3 ± 0.6 | 101.7 ± 11.5 | 12.3 ± 0.5 | 536.0 ± 31.4 |
| 625 | – | 123.3 ± 13.5 | 16.3 ± 4.5 | 101.7 ± 8.3 | 13.0 ± 2.6 | 541.3 ± 38.4 |
| 1250 | – | 128.3 ± 11.6 | 15.3 ± 5.8 | 109.0 ± 6.9 | 12.0 ± 3.0 | 494.7 ± 37.4 |
| 2500 | – | 114.0 ± 13.0 | 9.7 ± 1.5 | 103.0 ± 11.5 | 11.0 ± 3.0 | 480.7 ± 56.1 |
| 5000 | – | 112.0 ± 7.0 | 14.3 ± 1.1 | 104.0 ± 2.6 | 14.0 ± 3.0 | 432.0 ± 31.4 |
| Positive control | ||||||
| NPD | – | 524.7 ± 104.5 * | 836.0 ± 72.6 * | |||
| NaN3 | – | 1209.7 ± 263.3 * | 352.3 ± 45.6 * | |||
| MMC | – | 1970.7 ± 113.4 * | ||||
| Negative control | + | 138.3 ± 7.3 | 24.7 ± 1.5 | 122.0 ± 20.8 | 11.7 ± 1.5 | 503.0 ± 70.5 |
| 312.5 | + | 128.3 ± 16.0 | 27.0 ± 2.0 | 105.3 ± 8.1 | 12.0 ± 2.6 | 538.0 ± 14.0 |
| 625 | + | 136.0 ± 15.6 | 18.0 ± 4.3 | 123.0 ± 5.0 | 11.3 ± 4.6 | 516.7 ± 36.0 |
| 1250 | + | 152.3 ± 6.6 | 23.7 ± 1.5 | 121.7 ± 8.5 | 13.7 ± 0.5 | 510.7 ± 32.3 |
| 2500 | + | 123.3 ± 11.1 | 24.0 ± 3.4 | 108.0 ± 11.7 | 12.7 ± 3.2 | 485.3 ± 19.4 |
| 5000 | + | 128.0 ± 11.5 | 17.3 ± 4.9 | 111.3 ± 15.3 | 15.3 ± 2.5 | 452.0 ± 26.0 |
| Positive control | ||||||
| 2-AF | + | 367.3 ± 28.3 * | 287.3 ± 22.6 | |||
| BP | + | 59.7 ± 10.2 * | ||||
| 2-AA | + | 108.7 ± 15.5 * | 1009.3 ± 56.05 * | |||
| LMF-LJ (μg/mL) | S9 | Cell Viability (×106 cells) | Number of Aberrations | Aberrant Cell (% ± SD) a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG | TG | SB | SD | TB | TD | TR | QR | R | CR | DC | PP | PC | ||||
| Negative control b | – | 3.45 ± 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Positive control c | – | 2.78 ± 0.09 | 0 | 0 | 0 | 0 | 2 | 0 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 12.6 ± 1.1 * |
| 312.5 | – | 3.58 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 625 | – | 3.70 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 1250 | – | 3.85 ± 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 2500 | – | 3.63 ± 0.02 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 ± 0.5 |
| 5000 | – | 3.53 ± 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Negative control | + | 3.68 ± 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Positive control | + | 2.60 ± 0.12 | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 5 | 0 | 0 | 0 | 0 | 2 | 21.3 ± 1.4 * |
| 312.5 | + | 3.90 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 625 | + | 3.73 ± 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 1250 | + | 3.88 ± 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| 2500 | + | 3.58 ± 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1.2 ± 0.3 |
| 5000 | + | 3.80 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1.5 ± 1.0 |
| Sample | Dose (mg/kg BW) | Body Weight (g) | RETs/1000 Erythrocytes (%) | MNs/2000 RET (%) | Clinical Signs | Mortalities (Dead/Total) | |
|---|---|---|---|---|---|---|---|
| First treatment | Sacrifice | ND | 0/5 | ||||
| Negative control | 31.44 ± 1.49 a | 30.5 ± 1.9 | 30.5 ± 1.9 | 1.2 ± 0.8 | ND | 0/5 | |
| Positive control b | 1 | 30.50 ± 1.76 | 10.9 ± 4.6 * | 10.9 ± 4.6 * | 29.3 ± 5.4 * | ND | 0/5 |
| LMF | 500 | 31.70 ± 1.56 | 26.4 ± 3.2 | 26.4 ± 3.2 | 1.0 ± 0.7 | ND | 0/5 |
| 1000 | 31.64 ± 1.80 | 24.8 ± 3.9 | 24.8 ± 3.9 | 1.8 ± 1.3 | ND | 0/5 | |
| 2000 | 32.12 ± 1.43 | 28.5 ± 3.6 | 28.5 ± 3.6 | 1.4 ± 1.5 | ND | 0/5 | |
| Sex | Dose (mg/kg BW) | Volume (mL) | SG | pH | Protein (mg/dL) | Uro (EU/dL) | Glu | Bilirubin | Ketone a | Nit | Oc. Blood | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | ± | +1 | +2 | N | N | ± | |||||||
| Male | 0 | 6.7 ± 0.3 b | 1.024 ± 0.001 | 7.1 ± 0.1 | 86.2 ± 8.3 | 0.32 ± 0.03 | 10 c | 5 | 5 | 2 | 8 | 10 | 10 | 0 |
| 500 | 6.7 ± 0.8 | 1.019 ± 0.004 | 7.2 ± 0.4 | 97.7 ± 10.2 | 0.28 ± 0.02 | 10 | 4 | 6 | 3 | 7 | 10 | 10 | 0 | |
| 1000 | 6.4 ± 0.6 | 1.025 ± 0.002 | 7.0 ± 0.2 | 92.4 ± 9.6 | 0.30 ± 0.03 | 10 | 5 | 5 | 2 | 8 | 10 | 10 | 0 | |
| 2000 | 6.8 ± 0.2 | 1.024 ± 0.006 | 6.9 ± 0.1 | 98.1 ± 11.4 | 0.30 ± 0.01 | 10 | 5 | 5 | 1 | 9 | 10 | 9 | 1 | |
| Female | 0 | 5.9 ± 0.8 | 1.019 ± 0.005 | 7.2 ± 0.6 | 75.6 ± 6.5 | 0.26 ± 0.01 | 10 | 7 | 3 | 8 | 2 | 10 | 10 | 0 |
| 500 | 6.5 ± 0.3 | 1.021 ± 0.003 | 7.1 ± 0.4 | 83.1 ± 8.9 | 0.31 ± 0.08 | 10 | 6 | 4 | 3 | 7 | 10 | 10 | 0 | |
| 1000 | 5.8 ± 0.7 | 1.022 ± 0.007 | 6.8 ± 0.3 | 79.5 ± 10.0 | 0.30 ± 0.01 | 10 | 9 | 1 | 7 | 3 | 10 | 10 | 0 | |
| 2000 | 6.2 ± 0.4 | 1.027 ± 0.006 | 6.9 ± 0.2 | 89.2 ± 7.8 | 0.29 ± 0.02 | 10 | 7 | 3 | 6 | 4 | 10 | 10 | 0 | |
| Sex | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg BW) | 0 | 500 | 1000 | 2000 | 0 | 500 | 1000 | 2000 |
| RBC (M/μL) | 7.82 ± 0.19 a | 7.59 ± 0.26 | 7.87 ± 0.35 | 7.70 ± 0.15 | 7.90 ± 0.31 | 8.08 ± 0.34 | 8.10 ± 0.56 | 8.16 ± 0.55 |
| WBC (K/μL) | 8.96 ± 1.51 | 9.61 ± 0.94 | 10.43 ± 1.99 | 9.51 ± 1.38 | 6.85 ± 0.99 | 6.44 ± 1.97 | 6.04 ± 2.57 | 6.49 ± 1.68 |
| PLT (K/μL) | 1050.2 ± 66.7 | 1043.0 ± 102.0 | 1030.9 ± 93.8 | 957.9 ± 61.2 | 1025.9 ± 87.0 | 976.0 ± 108.9 | 1039.5 ± 77.2 | 1050.1 ± 109.1 |
| NEUT (%) | 16.97 ± 2.44 | 15.97 ± 5.30 | 14.29 ± 5.00 | 17.89 ± 5.58 | 14.82 ± 3.75 | 11.70 ± 4.57 | 10.11 ± 3.73 | 15.80 ± 6.70 |
| LYMPH (%) | 77.85 ± 2.75 | 80.17 ± 5.59 | 81.55 ± 5.44 | 76.90 ± 5.88 | 79.83 ± 3.97 | 80.17 ± 4.77 | 81.91 ± 3.67 | 79.87 ± 8.18 |
| PT (sec) | 14.12 ± 1.84 | 12.77 ± 0.97 | 13.46 ± 1.42 | 12.94 ± 0.97 | 9.97 ± 0.27 | 9.91 ± 0.12 | 9.84 ± 0.38 | 9.85 ± 0.14 |
| APTT (sec) | 18.13 ± 1.39 | 16.91 ± 1.06 | 17.29 ± 1.22 | 17.48 ± 1.01 | 15.33 ± 0.66 | 15.56 ± 0.84 | 16.35 ± 2.25 | 15.54 ± 0.27 |
| ALT (U/L) | 31.0 ± 4.1 | 32.5 ± 5.3 | 35.2 ± 8.5 | 35.8 ± 8.1 | 26.1 ± 3.0 | 29.1 ± 8.2 | 27.1 ± 6.6 | 31.0 ± 5.7 |
| AST (U/L) | 102.9 ± 17.6 | 106.3 ± 9.3 | 104.0 ± 17.3 | 118.3 ± 12.8 | 148.9 ± 21.7 | 130.5 ± 21.9 | 138.5 ± 21.3 | 146.7 ± 19.6 |
| ALP (U/L) | 171.6 ± 34.8 | 177.1 ± 28.6 | 181.2 ± 32.9 | 184.5 ± 25.2 | 98.4 ± 20.8 | 102.4 ± 25.8 | 101.3 ± 19.7 | 109.6 ± 21.02 |
| T-BIL (mg/dL) | 0.055 ± 0.016 | 0.045 ± 0.016 | 0.05.0 ± 0.000 | 0.05.0 ± 0.000 | 0.05.0 ± 0.000 | 0.05.0 ± 0.000 | 0.055 ± 0.016 | 0.05.0 ± 0.000 |
| TP (g/dL) | 6.46 ± 0.34 | 6.24 ± 0.30 | 6.46 ± 0.28 | 6.30 ± 0.24 | 6.93 ± 0.38 | 7.12 ± 0.46 | 6.90 ± 0.39 | 6.89 ± 0.62 |
| ALB (g/dL) | 4.12 ± 0.20 | 3.97 ± 0.13 | 4.04 ± 0.15 | 3.98 ± 0.14 | 4.42 ± 0.24 | 4.56 ± 0.28 | 4.38 ± 0.29 | 4.38 ± 0.35 |
| GLO (g/dL) | 2.34 ± 0.22 | 2.27 ± 0.18 | 2.42 ± 0.16 | 2.32 ± 0.13 | 2.51 ± 0.17 | 2.56 ± 0.18 | 2.52 ± 0.21 | 2.51 ± 0.29 |
| BUN (mg/dL) | 15.26 ± 2.42 | 15.00 ± 1.86 | 15.05 ± 1.18 | 15.57 ± 1.58 | 17.43 ± 2.77 | 16.45 ± 2.25 | 17.36 ± 2.64 | 17.60 ± 3.31 |
| CRE (mg/dL) | 0.54 ± 0.09 | 0.47 ± 0.02 * | 0.47 ± 0.05 * | 0.50 ± 0.04 | 0.62 ± 0.06 | 0.53 ± 0.06 * | 0.52 ± 0.04 * | 0.55 ± 0.04 * |
| TC (mg/dL) | 64.2 ± 13.9 | 66.3 ± 13.0 | 56.4 ± 7.4 | 61.5 ± 7.7 | 77.4 ± 15.6 | 74.7 ± 15.0 | 84.4 ± 19.5 | 67.8 ± 12.1 |
| TG (mg/dL) | 53.5 ± 10.4 | 37.9 ± 9.5 * | 35.8 ± 6.9 * | 41.4 ± 8.1 * | 46.4 ± 8.1 | 37.7 ± 13.4 * | 37.6 ± 10.8 * | 31.3 ± 9.4 * |
| Na (mmol/L) | 147.4 ± 1.0 | 146.2 ± 1.3 | 147.2 ± 1.5 | 147.2 ± 1.0 | 145.1 ± 1.4 | 145.7 ± 2.0 | 144.6 ± 2.1 | 146.1 ± 1.5 |
| K (mmol/L) | 7.21 ± 1.07 | 7.83 ± 0.71 | 7.71 ± 0.70 | 7.01 ± 1.06 | 7.38 ± 0.64 | 7.33 ± 0.51 | 7.23 ± 0.89 | 7.34 ± 1.36 |
| Ca (mg/dL) | 10.80 ± 0.36 | 11.08 ± 0.23 | 11.21 ± 0.50 | 11.15 ± 0.36 | 11.38 ± 0.39 | 11.67 ± 0.27 | 11.32 ± 0.43 | 11.51 ± 0.59 |
| P (mg/dL) | 13.32 ± 2.79 | 13.70 ± 0.90 | 14.03 ± 1.30 | 14.04 ± 1.00 | 13.09 ± 1.11 | 12.65 ± 1.04 | 12.00 ± 0.85 | 13.87 ± 1.28 |
| Sex | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg BW) | 0 | 500 | 1000 | 2000 | 0 | 500 | 1000 | 2000 | |
| Brain | Weight (g) | 1.99 ± 0.09 a | 1.95 ± 0.08 | 1.98 ± 0.07 | 1.95 ± 0.06 | 1.82 ± 0.07 | 1.75 ± 0.10 | 1.82 ± 0.05 | 1.81 ± 0.09 |
| Heart | Weight (g) | 1.39 ± 0.11 | 1.34 ± 0.07 | 1.36 ± 0.07 | 1.36 ± 0.09 | 0.81 ± 0.07 | 0.83 ± 0.08 | 0.82 ± 0.08 | 0.80 ± 0.03 |
| Ratio b | 0.69 ± 0.07 | 0.69 ± 0.03 | 0.69 ± 0.04 | 0.70 ± 0.05 | 0.44 ± 0.04 | 0.47 ± 0.03 | 0.45 ± 0.04 | 0.44 ± 0.02 | |
| Kidneys | Weight (g) | 3.09 ± 0.23 | 2.99 ± 0.24 | 2.97 ± 0.25 | 3.06 ± 0.29 | 1.66 ± 0.06 | 1.66 ± 0.18 | 1.56 ± 0.14 | 1.66 ± 0.12 |
| Ratio | 1.55 ± 0.14 | 1.52 ± 0.08 | 1.50 ± 0.16 | 1.57 ± 0.14 | 0.91 ± 0.05 | 0.95 ± 0.08 | 0.85 ± 0.07 | 0.92 ± 0.07 | |
| Liver | Weight (g) | 13.24 ± 1.71 | 12.45 ± 1.19 | 12.82 ± 0.64 | 12.66 ± 0.64 | 6.97 ± 0.61 | 7.29 ± 0.76 | 7.23 ± 0.89 | 6.97 ± 0.55 |
| Ratio | 6.65 ± 0.97 | 6.38 ± 0.67 | 6.48 ± 0.44 | 6.50 ± 0.39 | 3.82 ± 0.38 | 4.17 ± 0.39 | 3.96 ± 0.45 | 3.86 ± 0.34 | |
| Spleen | Weight (g) | 0.72 ± 0.11 | 0.70 ± 0.08 | 0.71 ± 0.06 | 0.74 ± 0.11 | 0.43 ± 0.06 | 0.42 ± 0.06 | 0.49 ± 0.08 | 0.42 ± 0.05 |
| Ratio | 0.36 ± 0.06 | 0.36 ± 0.04 | 0.36 ± 0.03 | 0.38 ± 0.06 | 0.23 ± 0.03 | 0.23 ± 0.02 | 0.26 ± 0.04 | 0.23 ± 0.02 | |
| Adrenals | Weight (g) | 0.050 ± 0.006 | 0.053 ± 0.004 | 0.055 ± 0.005 | 0.050 ± 0.008 | 0.060 ± 0.006 | 0.059 ± 0.007 | 0.058 ± 0.008 | 0.059 ± 0.008 |
| Ratio (%) c | 2.514 ± 0.347 | 2.733 ± 0.225 | 2.785 ± 0.281 | 2.579 ± 0.410 | 3.320 ± 0.380 | 3.386 ± 0.385 | 3.223 ± 0.463 | 3.271 ± 0.511 | |
| Testes | Weight (g) | 2.98 ± 0.16 | 3.13 ± 0.28 | 3.03 ± 0.31 | 2.92 ± 0.21 | - | - | - | - |
| Ratio | 1.49 ± 0.09 | 1.60 ± 0.15 | 1.53 ± 0.19 | 1.50 ± 0.09 | - | - | - | - | |
| Ovaries | Weight (g) | - | - | - | - | 0.067 ± 0.010 | 0.076 ± 0.008 | 0.074 ± 0.016 | 0.072 ± 0.013 |
| Ratio (%) | - | - | - | - | 3.680 ± 0.471 | 4.397 ± 0.562 | 4.072 ± 0.886 | 4.031 ± 0.822 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, P.-A.; Yan, M.-D.; Lin, H.-T.V.; Li, K.-L.; Lin, Y.-C. Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo. Mar. Drugs 2016, 14, 121. https://doi.org/10.3390/md14070121
Hwang P-A, Yan M-D, Lin H-TV, Li K-L, Lin Y-C. Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo. Marine Drugs. 2016; 14(7):121. https://doi.org/10.3390/md14070121
Chicago/Turabian StyleHwang, Pai-An, Ming-De Yan, Hong-Ting Victor Lin, Kuan-Lun Li, and Yen-Chang Lin. 2016. "Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo" Marine Drugs 14, no. 7: 121. https://doi.org/10.3390/md14070121
APA StyleHwang, P.-A., Yan, M.-D., Lin, H.-T. V., Li, K.-L., & Lin, Y.-C. (2016). Toxicological Evaluation of Low Molecular Weight Fucoidan in Vitro and in Vivo. Marine Drugs, 14(7), 121. https://doi.org/10.3390/md14070121