A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells
Abstract
:1. Introduction
2. Results
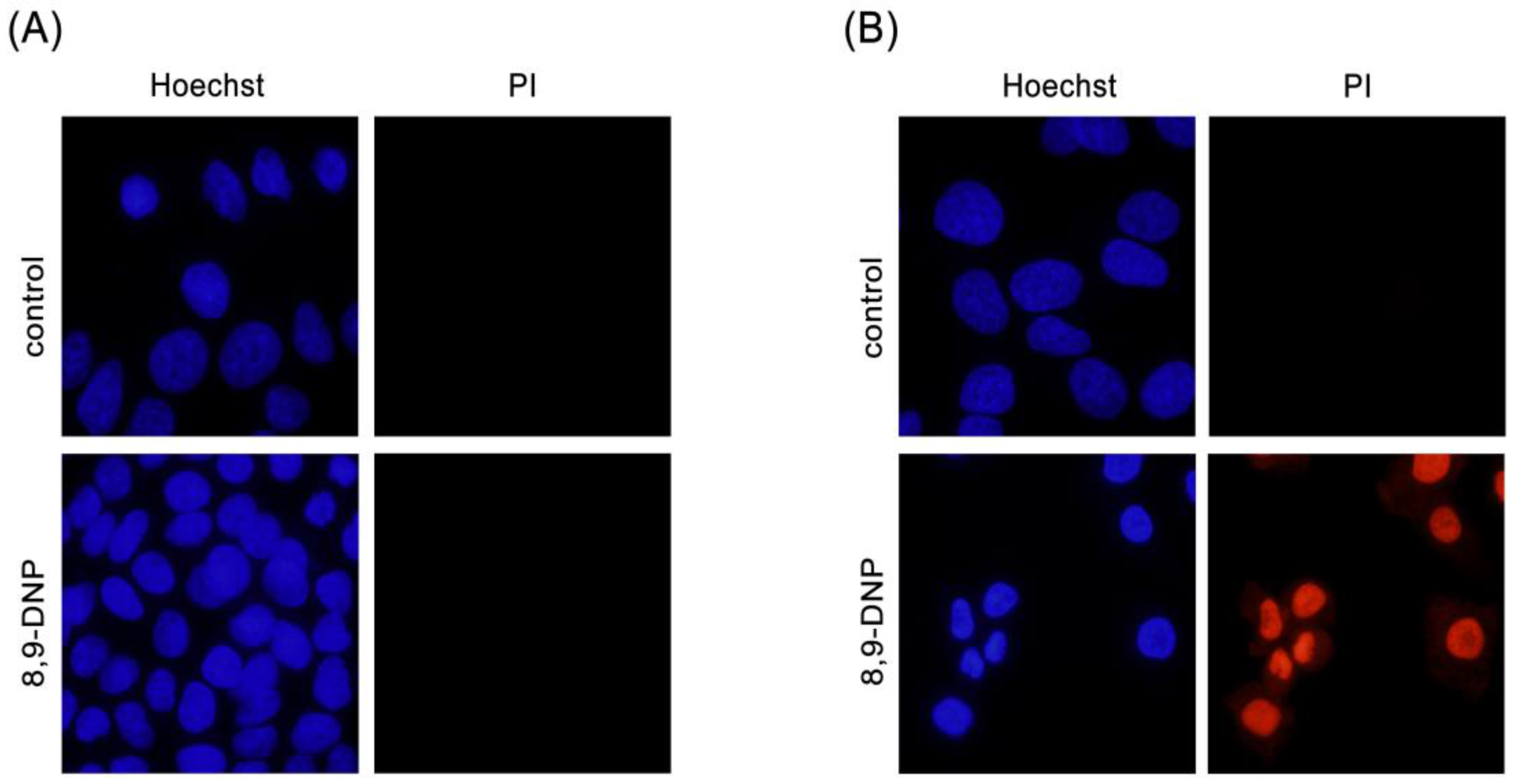
2.1. 8,9-DNP Shows Prefential Cytotoxicity in Starved Tumor Cells
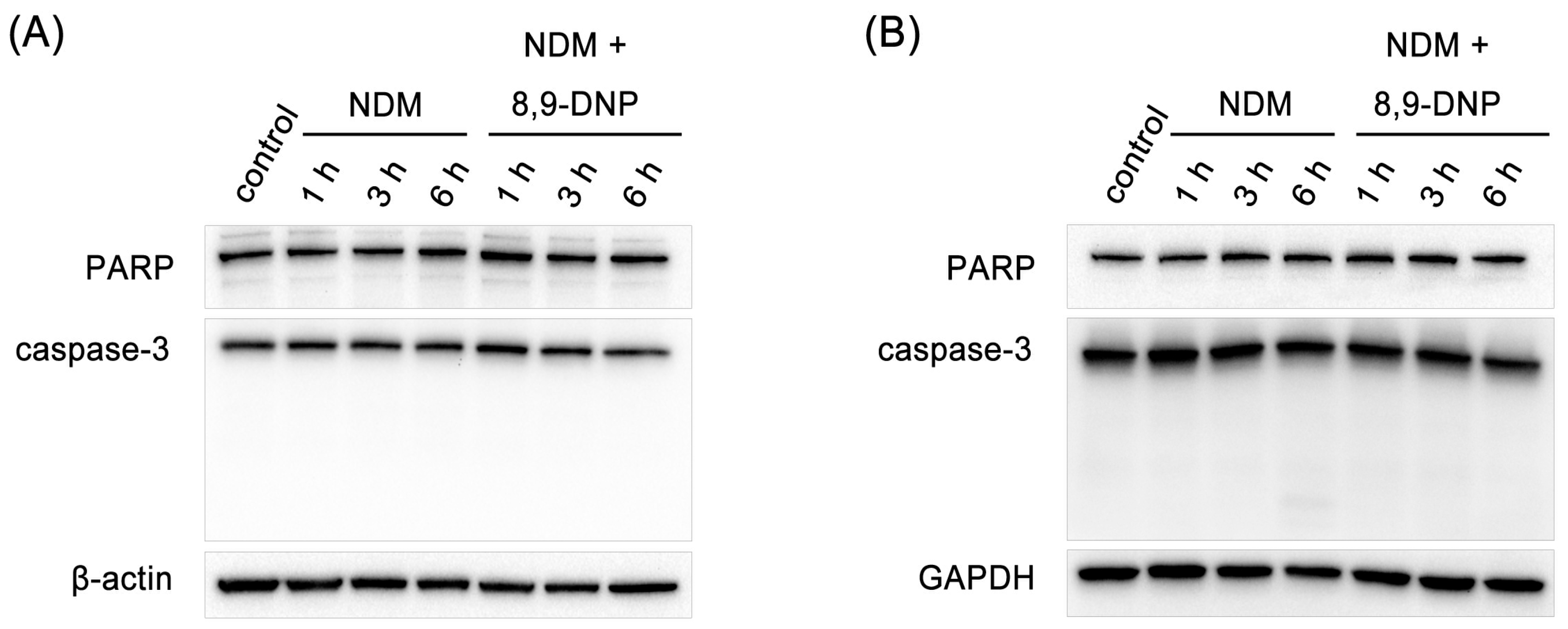
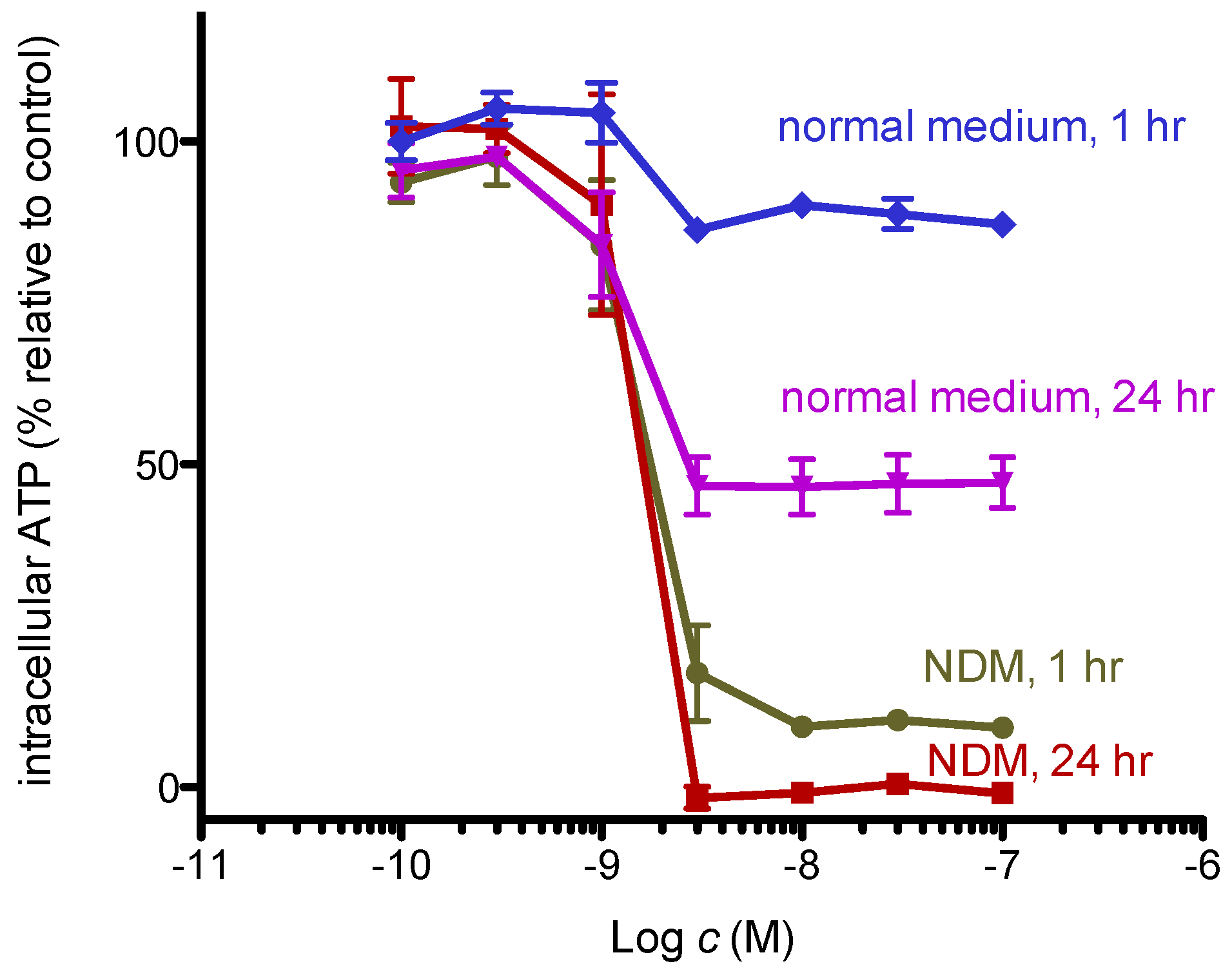
2.2. 8,9-DNP Dissipates the Mitochondrial Membrane Potential and Depletes Intracellular ATP Level in Starved Cells
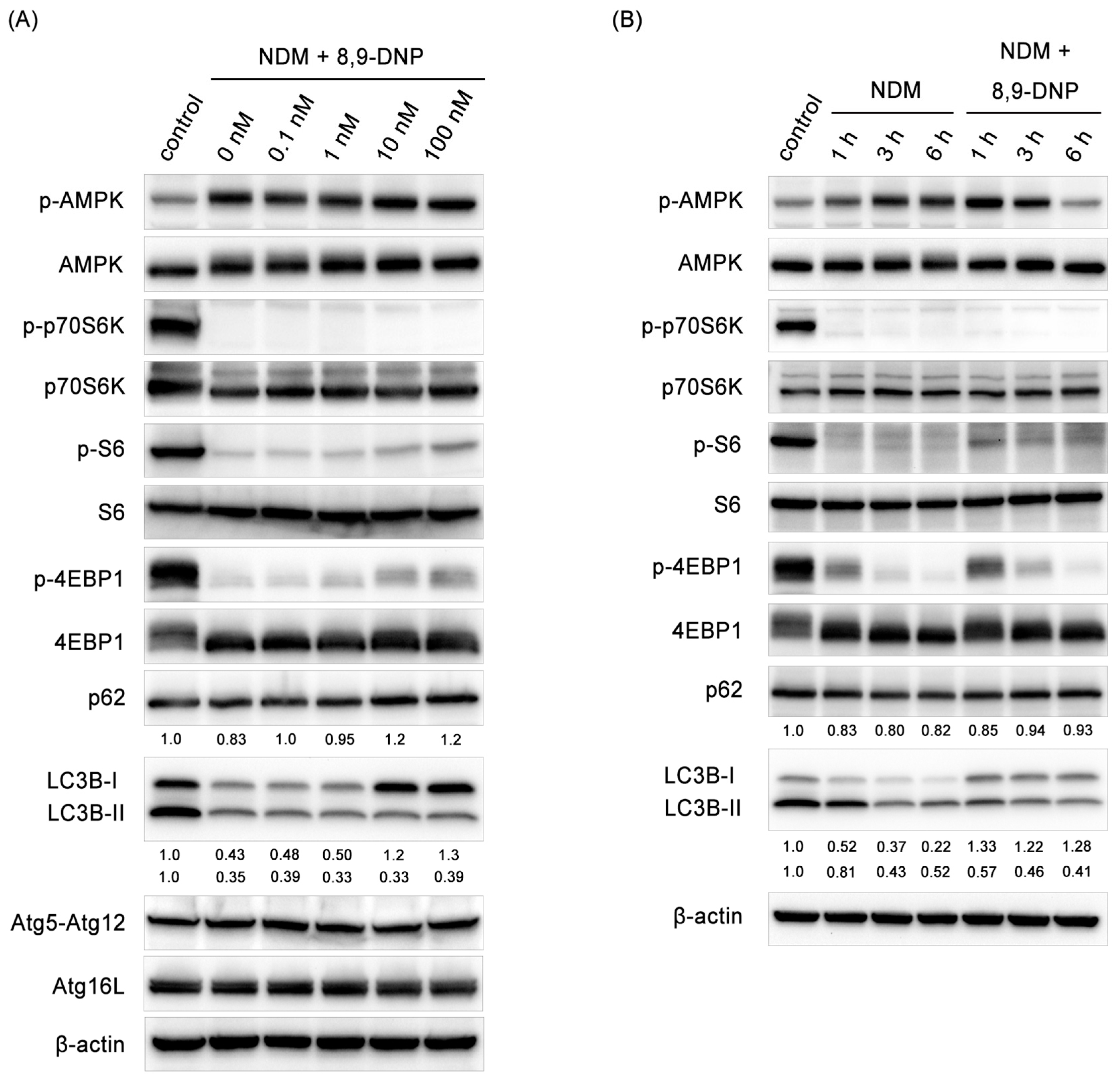
2.3. 8,9-DNP Inhibited Autophagic Flux in Starved Cells
2.4. Glucose Restores Autophagic Flux and Rescues Starved Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Viability Assay
4.3. Nuclear Morphology Analysis
4.4. Mitochondrial Membrane Potential Assay
4.5. Measurement of Intracellular ATP Concentration
4.6. Preparation of Cell Extract
4.7. Immunoblot Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Soga, T. Cancer metabolism: Key players in metabolic reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Izuishi, K.; Kato, K.; Ogura, T.; Kinoshita, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201–6207. [Google Scholar] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.F.; Renna, M.; Ghislat, G.; Puri, C.; Ashkenazi, A.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Mammalian autophagy: How does it work? Ann. Rev. Biochem. 2016, 85, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihara, K.; Fujii, S.; Esumi, H. Autophagy and cancer: Dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 2009, 278, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kunimoto, S.; Yamazaki, Y.; Kaminishi, M.; Esumi, H.; Kigamicin, D. A novel anticancer agent based on a new anti-austerity strategy targeting cancer cells’ tolerance to nutrient starvation. Cancer Sci. 2004, 95, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Lu, J.; Kalauni, S.K.; Kurashima, Y.; Tezuka, Y.; Kadota, S.; Esumi, H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006, 66, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, M.T.T.; Nguyen, H.X.; Dang, P.H.; Dibwe, D.F.; Esumi, H.; Awale, S. Constituents of the rhizomes of Boesenbergia pandurata and their antiausterity activities against the PANC-1 human pancreatic cancer line. J. Nat. Prod. 2017, 80, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Ishida, R.; Matsumoto, H.; Arai, M.; Toda, K.; Setiawan, A.; Muraoka, O.; Kobayashi, M. Biakamides A–D, unique polyketides from a marine sponge, act as selective growth inhibitors of tumor cells adapted to nutrient starvation. J. Org. Chem. 2017, 82, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
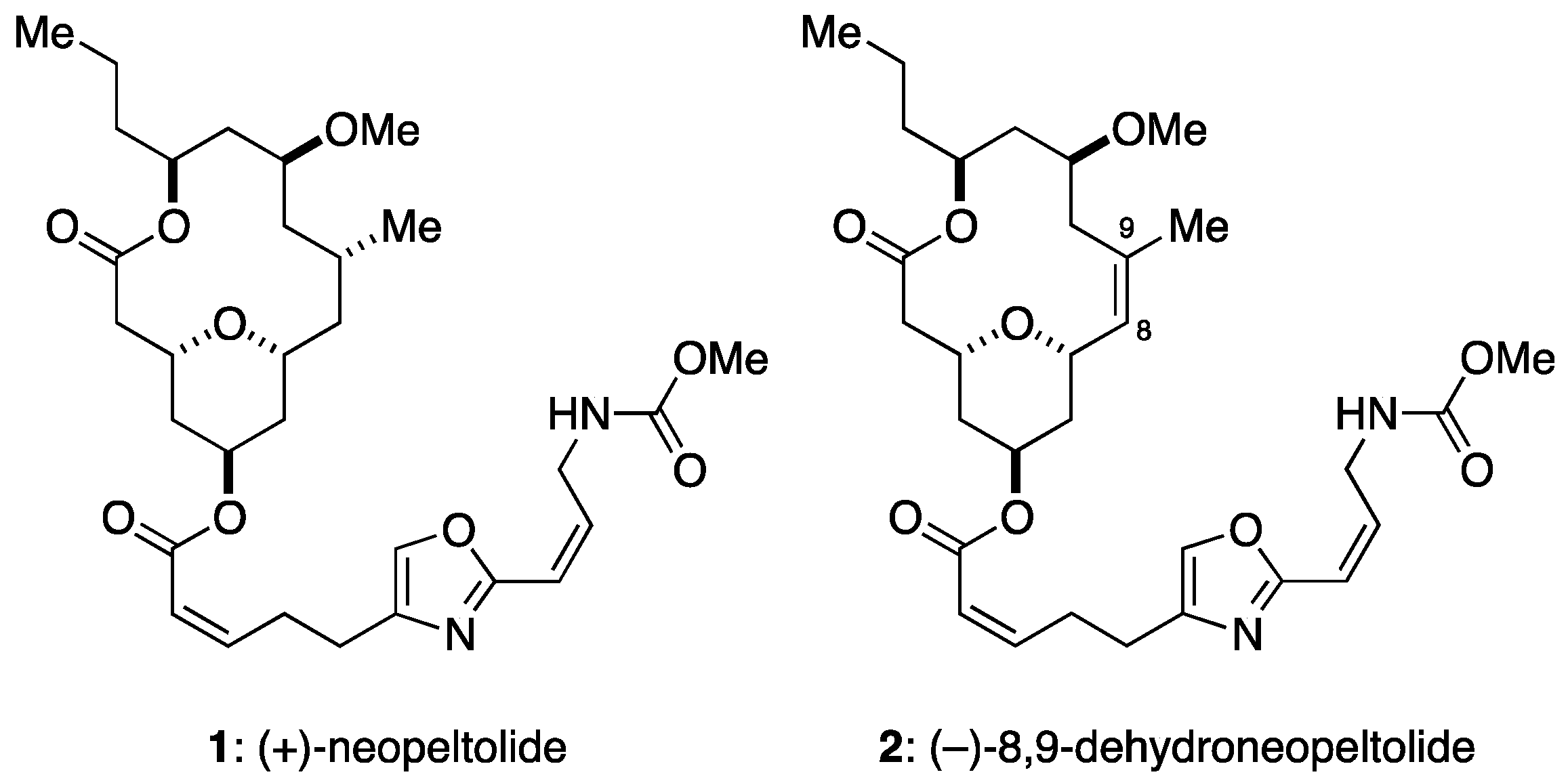
- Wright, A.E.; Botelho, J.C.; Guzmán, E.; Harmody, D.; Linley, P.; McCarthy, P.J.; Pitts, T.P.; Pomponi, S.A.; Reed, J.K. Neopeltolide, a macrolide from a lithistid sponge of the family Neopeltidae. J. Nat. Prod. 2007, 70, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H. Contemporary strategies for the synthesis of tetrahydropyran derivatives: Application to total synthesis of neopeltolide, a marine macrolide natural product. Mar. Drugs 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dai, M. Strategies and methods for the synthesis of anticancer natural product neopeltolide and its analogs. Curr. Org. Chem. 2015, 19, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Ulanovskaya, O.A.; Janjic, J.; Suzuki, M.; Sabharwal, S.S.; Schumacker, P.T.; Kron, S.J.; Kozmin, S.A. Synthesis enables identification of the cellular target of leucascandrolide A and neopeltolide. Nat. Chem. Biol. 2008, 4, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Naito, S.; Goto, T.; Sasaki, M. Total synthesis of (+)-neopeltolide. Angew. Chem. Int. Ed. 2008, 47, 4737–4739. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Saito, A.; Naito, S.; Konoki, K.; Yotsu-Yamashita, M.; Sasaki, M. Total synthesis and biological evaluation of (+)-neopeltolide and its analogues. Chem. Eur. J. 2009, 15, 12807–12818. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Saito, A.; Sasaki, M. A concise total synthesis of (+)-neopeltolide. Angew. Chem. Int. Ed. 2010, 49, 3041–3044. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Kawakami, M.; Noto, K.; Muto, T.; Suga, Y.; Konoki, K.; Yotsu-Yamashita, M.; Sasaki, M. Concise synthesis and biological assessment of (+)-neopeltolide and a 16-member stereoisomer library of 8,9-dehydroneopeltolide: Identification of pharmacophoric elements. Chem. Eur. J. 2013, 19, 8100–8110. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Noguchi, T.; Kawakami, M.; Sasaki, M. Synthesis and biological evaluation of (+)-neopeltolide analogues: Importance of the oxazole-containing side chain. Bioorg. Med. Chem. Lett. 2014, 24, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Sato, M.; Sasaki, M. Programmed cell death induced by (−)-8,9-dehydroneopeltolide in human promyelocytic leukemia HL-60 cells under energy stress conditions. Mar. Drugs 2014, 12, 5576–5589. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Ohba, S.; Tatsuda, D.; Kawada, M.; Masuda, T.; Tsujiuchi, G.; Yamori, T.; Esumi, H.; Ikeda, D. Mitochondrial inhibitors show preferential cytotoxicity to human pancreatic cancer PANC-1 cells under glucose-deprived conditions. Biochem. Biophys. Res. Commun. 2010, 392, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Reers, M.; Smith, T.W.; Chen, L.B. J-Aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef] [PubMed]
- Romanov, J.; Walczak, M.; Ibiricu, I.; Schüchner, S.; Ogris, E.; Kraft, C.; Martens, S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012, 31, 4304–4317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, H.-J.; Jeong, H.K.; Kim, M.-J.; Kim, M.; Bae, O.-N.; Baek, S.-H. Autophagy sustains the survival of human pancreatic cancer PANC-1 cells under extreme nutrient deprivation conditions. Biochem. Biophys. Res. Commun. 2015, 463, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Imamura, Y.; Emoto, K.; Umeda, M.; Noda, T.; Ohsumi, Y. In vivo and in vitro reconstitution of Atg8 conjugation essential for autophagy. J. Biol. Chem. 2004, 279, 40584–40592. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Rad. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Kunimoto, S.; Osono, M.; Ikeda, D. Inhibitors of insulin-like growth factor-1 receptor tyrosine kinase are preferentially cytotoxic to nutrient-deprived pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2009, 380, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Athikomkulchai, S.; Miyatake, R.; Saiki, I.; Esumi, H.; Awale, S. (+)-Grandifloracin, an antiausterity agent, induces autophagic PANC-1 pancreatic cancer cell death. Drug Des. Dev. Ther. 2014, 8, 39–47. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Moruno-Manchón, J.F.; Pérez-Jiménez, E.; Knecht, E. Glucose induces autophagy under starvation conditions by a p38 MAPK-dependent pathway. Biochem. J. 2013, 449, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.L.; Tooze, S.A. Coordinated regulation of autophagy by p38α MAPK through mAtg9 and p38IP. EMBO J. 2010, 29, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuwa, H.; Sato, M. A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells. Mar. Drugs 2017, 15, 320. https://doi.org/10.3390/md15100320
Fuwa H, Sato M. A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells. Marine Drugs. 2017; 15(10):320. https://doi.org/10.3390/md15100320
Chicago/Turabian StyleFuwa, Haruhiko, and Mizuho Sato. 2017. "A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells" Marine Drugs 15, no. 10: 320. https://doi.org/10.3390/md15100320
APA StyleFuwa, H., & Sato, M. (2017). A Synthetic Analogue of Neopeltolide, 8,9-Dehydroneopeltolide, Is a Potent Anti-Austerity Agent against Starved Tumor Cells. Marine Drugs, 15(10), 320. https://doi.org/10.3390/md15100320




