Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Materials
2.3. Patient Selection
2.4. Randomization and Blinding
2.5. Study Protocols
2.6. Efficacy Objectives
2.6.1. Primary Objective
2.6.2. Secondary Objectives
2.6.3. Safety Objective
2.7. Length of Study
2.8. Efficacy Outcome Measures
2.9. Statistical Analysis
3 Results
3.1. Baseline Characteristics of Patients
3.2. Primary Outcome
3.3. Secondary Outcomes
3.4. Evaluation of Adverse Effects
3.5. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moussavou, G.; Kwak, D.H.; Obiang-Obonou, B.W.; Maranguy, C.A.; Dinzouna-Boutamba, S.D.; Lee, D.H.; Pissibanganga, O.G.; Ko, K.; Seo, J.I.; Choo, Y.K. Anticancer effects of different seaweeds on human colon and breast cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kusaykin, M.I.; Kurilenko, V.V.; Zakharenko, A.M.; Isakov, V.V.; Zaporozhets, T.S.; Gazha, A.K.; Zvyagintseva, T.N. Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, Formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ye, X.; Sun, Y.; Wu, D.; Wu, N.; Hu, Y.; Chen, S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested Fucoidan Extracts Derived from Seaweed Mozuku of Cladosiphon novae-caledoniae kylin Inhibit Invasion and Angiogenesis of Tumor Cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, D. Amzaing Power of Fucoidan (You Can Beat Cancer Too!!); Dacombook: Taipei, Taiwan, 2009. [Google Scholar]
- Chen, W. Qi Ji Yi Sheng Chen Wei Hua 20 Nian Zhan Sheng 3 Ai; Fang Zhou Mu Ma Wen Hua: Xinbei, Taiwan, 2013. [Google Scholar]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients With Breast Cancer. Integr. Cancer Ther. 2016. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chou, T.C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Tseng, L.M.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFbeta receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFβ receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.D.; Yao, C.J.; Chow, J.M.; Chang, C.L.; Hwang, P.A.; Chuang, S.E.; Whang-Peng, J.; Lai, G.M. Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2017. [Google Scholar] [CrossRef]
- Taiwan Cancer Registry. Available online: http://tcr.cph.ntu.edu.tw/main.php?Page=A5B2 (accessed on 7 December 2016).
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Haugan, K.; Karunakaran, P.; Blatny, J.M.; Valla, S. The phenotypes of temperature-sensitive mini-RK2 replicons carrying mutations in the replication control gene trfA are suppressed nonspecifically by intragenic cop mutations. J. Bacteriol. 1992, 174, 7026–7032. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 7 December 2016).
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Qiu, H.; Zhang, M.; Sun, L.; Peng, P.; Yu, Q.; Yuan, X. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2017, 8, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Troiani, T.; Napolitano, S.; Della Corte, C.M.; Martini, G.; Martinelli, E.; Morgillo, F.; Ciardiello, F. Therapeutic value of EGFR inhibition in CRC and NSCLC: 15 years of clinical evidence. ESMO Open 2016, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Meropol, N.J.; Loehrer, P.J., Sr.; Needle, M.N.; Kopit, J.; Mayer, R. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin. Oncol. 2004, 22, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.L.; Van Laethem, J.L.; Maurel, J.; Richardson, G.; et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Hsu, H.C.; Thiam, T.K.; Lu, Y.J.; Yeh, C.Y.; Tsai, W.S.; You, J.F.; Hung, H.Y.; Tsai, C.N.; Hsu, A.; Chen, H.C.; et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget 2016, 7, 22257–22270. [Google Scholar] [PubMed]
- Bos, J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar] [PubMed]
- Fernandez-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.; Li, S.; Liu, T.; Wang, F.; Xia, Y.; Lu, J.; Zhou, Y.; Guo, C. Pretreatment with Fucoidan from Fucus vesiculosus Protected against ConA-Induced Acute Liver Injury by Inhibiting Both Intrinsic and Extrinsic Apoptosis. PLoS ONE 2016, 11, e0152570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.; Li, S.; Feng, J.; Liu, T.; Wang, F.; Zhang, R.; Xu, S.; Zhou, Y.; Zhou, S.; et al. Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-beta1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. Dev. Ther. 2016, 10, 619–630. [Google Scholar]
- Jia, Y.; Sun, Y.; Weng, L.; Li, Y.; Zhang, Q.; Zhou, H.; Yang, B. Low molecular weight fucoidan protects renal tubular cells from injury induced by albumin overload. Sci. Rep. 2016, 6, 31759. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, W.; Zhang, Q.; Jia, Y.; Sun, Y.; Weng, L.; Luo, D.; Zhou, H.; Yang, B. Low molecular weight fucoidan ameliorates diabetic nephropathy via inhibiting epithelial-mesenchymal transition and fibrotic processes. Am. J. Transl. Res. 2015, 7, 1553–1563. [Google Scholar] [PubMed]
- Zuo, T.; Li, X.; Chang, Y.; Duan, G.; Yu, L.; Zheng, R.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, G.; Zhao, Y.T. Fucoidan attenuates the existing allodynia and hyperalgesia in a rat model of neuropathic pain. Neurosci. Lett. 2014, 571, 66–71. [Google Scholar] [CrossRef] [PubMed]



| Total | Study Group | Control Group | ||
|---|---|---|---|---|
| (n = 54) | (n = 28) | (n = 26) | p-value | |
| N | N (%) | N (%) | ||
| Gender | 0.967 # | |||
| Male | 31 | 16 (57.1) | 15 (57.7) | |
| Female | 23 | 12 (42.9) | 11 (42.3) | |
| Age (y/o) * | 0.178 # | |||
| <65 | 36 | 21 (75.0) | 15 (57.7) | |
| ≥65 | 18 | 7 (25.0) | 11 (42.3) | |
| Age (y/o) * | 0.137 ## | |||
| Median ± S.D * | 57.46 ± 12.15 | 62.38 ± 11.72 | ||
| (range) | (30 ~ 79) | (43 ~ 83) | ||
| Stage IV | 0.872 # | |||
| Synchronous | 40 | 21 (75.0) | 19 (73.1) | |
| Metachronous | 14 | 7 (25.0) | 7 (26.9) | |
| Metastasectomy | 0.244 # | |||
| Yes | 12 | 8 (28.6) | 4 (15.4) | |
| No | 42 | 20 (71.4) | 22 (84.6) | |
| Follow-up (months) | 0.117 ## | |||
| Median ± S.D * | 12.39 ± 4.41 | 10.54 ± 3.22 | ||
| (range) | (4 ~ 20) | (4 ~ 16) | ||
| Pre-WBC ** (/μL) | 0.671 ## | |||
| Mean ± S.D * | 7118 ± 2669 | 7330 ± 2960 | ||
| Median | 7065 | 7045 | ||
| Pre-Hgb ** (g/dL) | 0.472 ## | |||
| Mean ± S.D * | 11.89 ± 1.78 | 11.51 ± 1.90 | ||
| Median | 11.80 | 11.45 | ||
| Pre-Platelet (/μL) | 0.952 ## | |||
| Mean ± S.D * | 303,964 ± 99,869 | 300,346 ± 87,945 | ||
| Median | 278,500 | 292,500 | ||
| Pre-GPT ** (U/L) | 0.646 ## | |||
| Mean ± S.D * | 23.54 ± 12.56 | 20.92 ± 6.80 | ||
| Median | 21.50 | 20.50 | ||
| Pre-Cr ** (mg/dL) | 0.591 ## | |||
| Mean ± S.D * | 0.96 ± 0.75 | 0.89 ± 0.26 | ||
| Median | 0.815 | 0.835 | ||
| Pre-CEA ** (ng/mL) | 0.236 ## | |||
| Mean ± S.D * | 989.9 ± 3622.5 | 35.05 ± 60.78 | ||
| Median | 31.38 | 13.92 | ||
| Pre-Body weight (kg) | 0.382 ## | |||
| Mean ± S.D * | 62.39 ± 11.30 | 59.50 ± 11.90 | ||
| Median | 61.45 | 58.00 |
| Total | Study Group | Control Group | ||
|---|---|---|---|---|
| (n = 54) | (n = 28) | (n = 26) | p-value | |
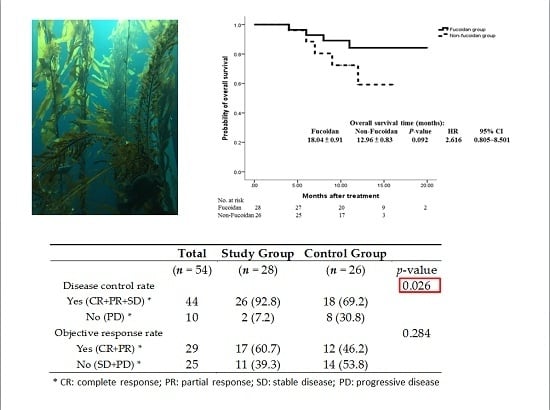
| Disease control rate | 0.026 | |||
| Yes (CR+PR+SD) * | 44 | 26 (92.8) | 18 (69.2) | |
| No (PD) * | 10 | 2 (7.2) | 8 (30.8) | |
| Objective response rate | 0.284 | |||
| Yes (CR+PR) * | 29 | 17 (60.7) | 12 (46.2) | |
| No (SD+PD) * | 25 | 11 (39.3) | 14 (53.8) |
| Study Group (n = 28) | Control Group (n = 26) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | p-value * | |
| WBC change (/μL) | −4508 | 2444 | −4435 | −4647.69 | 2821 | −4390 | 0.8558 |
| Hgb change (g/dL) | −3.00 | 1.80 | −2.95 | −2.49 | 1.77 | −2.25 | 0.3451 |
| Platelet change (/μL) | −144,750 | 120,530 | −92,000 | −132,808 | 86,573 | −124,000 | 0.8152 |
| GPT change (U/L) | 75.25 | 122.61 | 40.50 | 58.73 | 90.57 | 29.00 | 0.5974 |
| Creatinine change (mg/dL) | 0.52 | 0.86 | 0.16 | 0.69 | 1.52 | 0.16 | 0.2354 |
| CEA change (ng/mL) | −518.90 | 2472 | −12.31 | 1.41 | 111.32 | −5.56 | 0.4208 |
| Body weight change (kg) | −3.71 | 4.27 | −2.20 | −3.76 | 4.60 | −2.55 | 0.9035 |
| Study Group (n = 28) (%) | Control Group (n = 26) (%) | Study Group (n = 28) (%) | Control Group (n = 26) (%) | |||
|---|---|---|---|---|---|---|
| Grade | I-IV | I-IV | p-value | III & IV | III & IV | p-value |
| Leukopenia | 18 (64.3%) | 17 (65.4%) | 0.9327 | 7 (25%) | 7 (26.9%) | 0.8719 |
| Anemia | 22 (78.6%) | 17 (65.4%) | 0.2797 | 8 (28.6%) | 8 (30.8%) | 0.8597 |
| Thrombocytopenia | 7 (25%) | 4 (15.4%) | 0.3807 | 1 (3.6%) | 2 (7.7%) | 0.5089 |
| Abnormal liver function | 14 (50%) | 15 (57.7%) | 0.5710 | 5 (17.9%) | 4 (15.4%) | 0.8075 |
| Impaired renal function | 12 (42.9%) | 6 (23.1%) | 0.1234 | 1 (3.6%) | 4 (15.4%) | 0.1346 |
| Mucositis oral | 14 (50%) | 17 (65.4%) | 0.2533 | 1 (3.6%) | 1 (3.8%) | 0.9574 |
| Pruritus | 10 (35.7%) | 14 (53.9%) | 0.1803 | 0 | 0 | |
| Vomiting | 10 (35.7%) | 14 (53.9%) | 0.1803 | 0 | 3 (11.5%) | 0.0644 |
| Taste problem | 18 (64.3%) | 21 (80.8%) | 0.1766 | 2 (7.1%) | 2 (7.7%) | 0.9386 |
| Bloody stool | 4 (14.29%) | 8 (30.77%) | 0.1454 | 0 | 1 (3.85%) | 0.2948 |
| Alopecia | 26 (92.9%) | 25 (96.1%) | 0.5971 | 10 (35.7%) | 9 (34.6%) | 0.9326 |
| Study Group (n = 28) | Control Group (n = 26) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grading | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | p-value |
| Limited in doing daily activities | 0 | 12 | 13 | 3 | 0 | 10 | 10 | 6 | 0.5142 |
| Limited in doing hobbies | 4 | 15 | 6 | 3 | 0 | 11 | 13 | 2 | 0.0553 |
| Limited in walking | 9 | 14 | 3 | 2 | 6 | 9 | 9 | 2 | 0.2197 |
| Trouble sleeping | 7 | 12 | 6 | 3 | 4 | 5 | 14 | 3 | 0.0784 |
| Depression | 7 | 15 | 6 | 0 | 1 | 16 | 8 | 1 | 0.0971 |
| Anxiety | 6 | 12 | 9 | 1 | 3 | 12 | 11 | 0 | 0.5826 |
| Fatigue | 3 | 12 | 11 | 2 | 0 | 11 | 12 | 3 | 0.4520 |
| Feel weakness | 1 | 15 | 9 | 3 | 0 | 9 | 14 | 3 | 0.3108 |
| Need help with personal hygiene | 18 | 5 | 4 | 1 | 12 | 10 | 2 | 2 | 0.3031 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-L.; Tai, C.-J.; Huang, C.-W.; Chang, F.-R.; Wang, J.-Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial . Mar. Drugs 2017, 15, 122. https://doi.org/10.3390/md15040122
Tsai H-L, Tai C-J, Huang C-W, Chang F-R, Wang J-Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial . Marine Drugs. 2017; 15(4):122. https://doi.org/10.3390/md15040122
Chicago/Turabian StyleTsai, Hsiang-Lin, Chi-Jung Tai, Ching-Wen Huang, Fang-Rong Chang, and Jaw-Yuan Wang. 2017. "Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial " Marine Drugs 15, no. 4: 122. https://doi.org/10.3390/md15040122
APA StyleTsai, H.-L., Tai, C.-J., Huang, C.-W., Chang, F.-R., & Wang, J.-Y. (2017). Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial . Marine Drugs, 15(4), 122. https://doi.org/10.3390/md15040122