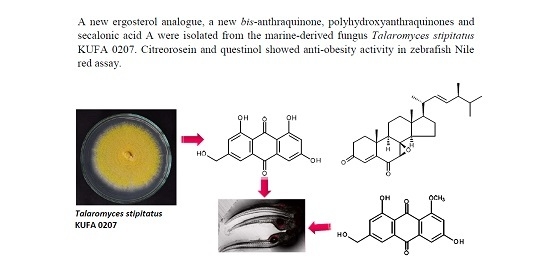
A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.3.1. Talarosterone (1): (7R,8R)-Epoxyergosta-4,22-dien-3,6-dione
3.3.2. Bis(1,4,5-Trihydroxy-7-methylanthraquinone) (3)
3.4. X-ray Crystal Structure of Compounds 1 and 20
3.5. Anti-Obesity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.M.; Li, J.; Jiang, C.X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; May Zin, W.W.; Dethoup, T.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Kijjoa, A. Secondary Metabolites from the Culture of the Marine Sponge-Associated Fungi Talaromyces tratensis and Sporidesmium circinophorum. Planta Med. 2016, 82, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Krishna, M.M.; Ishida, K.; Anjaneyulu, V. Marine sterols. XXII. Occurrence of 3-oxo-4, 6, 8(14)-triunsaturated steroids in the sponge Dysidea herbacea. Chem. Pharm. Bull. 1992, 40, 72–74. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Franzblau, S.G.; Fischer, N.H. Antimycobacterial plant terpenoids. Planta Med. 2001, 67, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Sekita, S.; Satake, M. Steroids from Calvatia cyathiformis. Phytochemistry 1994, 37, 213–215. [Google Scholar] [CrossRef]
- Bao, J.; Sun, Y.L.; Zhang, X.Y.; Han, Z.; Gao, H.C.; He, F.; Qian, P.Y.; Qi, S.H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. 2013, 66, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.C.; Li, Y.; Kim, E.A.; Kang, S.M.; Jeon, Y.J. Anti-inflammatory activity of questinol isolated from marine-derived fungus Eurotium amstelodami in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Microbiol. Biotechnol. 2014, 24, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T. The coloring matters of Xanthoria fallax (Hepp.) Arn. Fallacinal and fallacinol. Pharm. Bull. 1956, 4, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Stodůlková, E.; Kolarík, M.; Kresinová, Z.; Kuzma, M.; Sulc, M.; Man, P.; Novák, P.; Marsík, P.; Landa, P.; Olsovská, J.; et al. Hydroxylated anthraquinones produced by Geosmithia species. Folia Microbiol. 2009, 54, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Maebayashi, Y.; Miyaki, K. The isolation of secalonic acid A from Aspergillus ochraceus cultured on rice. Chem. Pharm. Bull. 1971, 19, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Porco, J.A., Jr. Total syntheses of secalonic acids A and D. Angew. Chem. Int. Ed. 2014, 53, 3107–3110. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Obesity: The metabolic disease, advances on drug discovery and natural product research. Curr. Top. Med. Chem. 2016, 16, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Anti-obesity Product and Its Method of Preparation. Available online: http://www.google.com/patents/US8247001 (access on 27 March 2017).
- Tzeng, T.F.; Lu, H.J.; Liou, S.S.; Chang, C.J.; Liu, I.M. Emodin, a naturally occurring anthraquinone derivative, ameliorates dyslipidemia by activating AMP-activated protein kinase in high-fat-diet-fed rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 781812. [Google Scholar] [CrossRef] [PubMed]
- Giacomotto, J.; Ségalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Alimov, A.P.; Rilo, H.L.; Jandacek, R.J.; Woollett, L.A.; Penberthy, W.T. A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr. Metab. 2008, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Cai, X.-L.; Wang, S.-Y.; Pan, J.-H.; Tao, Y.-W.; She, Z.-G.; Zhou, S.-N.; Lin, Y.-C.; Vrijmoed, L.L.P. A new hTopo I isomerase inhibitor produced by a mangrove endophytic fungus no. 2240. J. Asian Nat. Prod. Res. 2008, 10, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the constituents of Aloe saponaria Haw. II. The structures of tetrahydroanthracene derivatives aloesaponol III and IV. Chem. Pharm. Bull. 1977, 25, 1764–1770. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, J.S.; Feng, Z.M.; Zhang, P.C. Anthraquinone-benzisochromanquinone dimers from the roots of Berchemia floribunda. Chem. Pharm. Bull. 2008, 56, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- May Zin, W.W.; Buttachon, S.; Buaruang, J.; Gales, L.; Pereira, J.A.; Pinto, M.M.M.; Silva, A.M.S.; Kijjoa, A. A New Meroditerpene and a New Tryptoquivaline from the Algicolous Fungus Neosartorya takakii KUFC 7898. Mar. Drugs 2015, 13, 3776–3790. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

 ) and HMBC (
) and HMBC (  ) correlations of compound 1.
) correlations of compound 1.
 ) of compound 1.
) of compound 1.


 ) and HMBC (
) and HMBC (  ) correlations of compound 3.
) correlations of compound 3.


| Position | δC, Type | δH, (J in Hz) | COSY | ROESY | HMBC |
|---|---|---|---|---|---|
| 1α | 37.1, CH2 | 1.81, m | |||
| β | 2.18, m | ||||
| 2α | 33.8, CH2 | 2.50,m | H-1 | ||
| β | 2.64, dd (17.9, 7.2) | H-1 | |||
| 3 | 199.5, CO | - | |||
| 4 | 129.2, CH | 6.66, d (0.8) | - | C-2, 3, 5 | |
| 5 | 155.0, C | - | |||
| 6 | 195.9, CO | - | |||
| 7 | 56.1, CH | 3.20, s | - | H-9, 14, 15 | C-5, 6, 8, 14 |
| 8 | 66.2, C | - | |||
| 9 | 43.9, CH | 1.90, m | |||
| 10 | 36.8, C | - | |||
| 11 | 20.7, CH2 | 1.85, m | |||
| 12 | 39.0, CH2 | 1.21, m; 1.40, m | |||
| 13 | 44.3, C | - | |||
| 14 | 51.8, CH | 1.94, m | |||
| 15 | 18.7, CH2 | 1.25, m | |||
| 16 | 27.7, CH2 | 1.34, m; 1.75, m | |||
| 17 | 56.0, CH | 1.30, m | |||
| 18 | 12.4, CH3 | 0.89, s | C-12, 13, 14, 17 | ||
| 19 | 21.8, CH3 | 1.50, s | - | 1β, 2β | C-1, 5, 9 |
| 20 | 40.0, CH | 2.04, m | |||
| 21 | 21.0 CH3 | 1.04, d (6.8) | H-20 | C-17, 20, 22 | |
| 22 | 135.0, CH | 5.15, dd (15.3, 7.3) | H-20, 23 | C-21, 24 | |
| 23 | 132.5, CH | 5.25, dd (15.3, 8.0) | H-22, 24 | C-20, 24, 25, 26 | |
| 24 | 42.8, CH | 1.85, m | C-22, 23 | ||
| 25 | 33.0, CH | 1.48, m | |||
| 26 | 17.6, CH3 | 0.92, d (6.8) | H-24 | C-23, 24, 25 | |
| 27 | 20.0, CH3 | 0.82, d (6.8) | H-25 | C-24, 25, 28 | |
| 28 | 19.6 CH3 | 0.84, d (6.8) | H-25 | C-24, 25, 27 |
| Position | δC, Type | δH, (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 (1′) | 164.3, C | - | ||
| 2 (2′) | 123.5, C | - | ||
| 3 (3′) | 107.2, CH | 6.73, s | - | C-1 (1′), 4 (4′), 2 (2′), 4a (4′a) |
| 4 (4′) | 108.9, C | - | ||
| 4a (4′a) | 108.9, C | - | ||
| 5 (5′) | 161.1, C | - | ||
| 6 (6′) | 123.6, CH | 7.15, dd (1.0, 0.5) | H-8 (8′), Me-7 (7′) | C-5 (5′), 7 (7′), 8 (8′), 10a (10′a) |
| 7 (7′) | 148.2, C | - | ||
| 8 (8′) | 120.5, CH | 7.28, dd (1.0, 0.5) | H-6 (6′), Me-7 (7′) | C-6 (6′), 7 (7′), 9 (9′), 10a (10′a) |
| 8a (8′a) | 131.3, C * | - | ||
| 9 (9′) | 182.1, CO | - | ||
| 9a (9′a) | 133.2, C * | - | ||
| 10 (10′) | 189.6, CO | - | ||
| 10a (10′a) | 113.1, C | - | ||
| CH3-7 (7′) | 21.5, CH3 | 2.33, s | H-6 (6′), 8 (8′) | C-6 (6′), 7 (7′), 8 (8′) |
| OH-4 (4′) | - | 12.79, s | - | C-3 (3′), 4 (4′), 4a (4′a) |
| OH-5 (5′) | - | 12.04, s | - | C-5 (5′), 6 (6′), 10a (10′a) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noinart, J.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Urbatzka, R.; Freitas, S.; Lee, M.; Silva, A.M.S.; Pinto, M.M.M.; et al. A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207. Mar. Drugs 2017, 15, 139. https://doi.org/10.3390/md15050139
Noinart J, Buttachon S, Dethoup T, Gales L, Pereira JA, Urbatzka R, Freitas S, Lee M, Silva AMS, Pinto MMM, et al. A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207. Marine Drugs. 2017; 15(5):139. https://doi.org/10.3390/md15050139
Chicago/Turabian StyleNoinart, Jidapa, Suradet Buttachon, Tida Dethoup, Luís Gales, José A. Pereira, Ralph Urbatzka, Sara Freitas, Michael Lee, Artur M. S. Silva, Madalena M. M. Pinto, and et al. 2017. "A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207" Marine Drugs 15, no. 5: 139. https://doi.org/10.3390/md15050139
APA StyleNoinart, J., Buttachon, S., Dethoup, T., Gales, L., Pereira, J. A., Urbatzka, R., Freitas, S., Lee, M., Silva, A. M. S., Pinto, M. M. M., Vasconcelos, V., & Kijjoa, A. (2017). A New Ergosterol Analog, a New Bis-Anthraquinone and Anti-Obesity Activity of Anthraquinones from the Marine Sponge-Associated Fungus Talaromyces stipitatus KUFA 0207. Marine Drugs, 15(5), 139. https://doi.org/10.3390/md15050139