Cereus sinensis Polysaccharide and Its Immunomodulatory Properties in Human Monocytic Cells
Abstract
:1. Introduction
2. Results
2.1. Optimization of the Polysaccharide Extraction
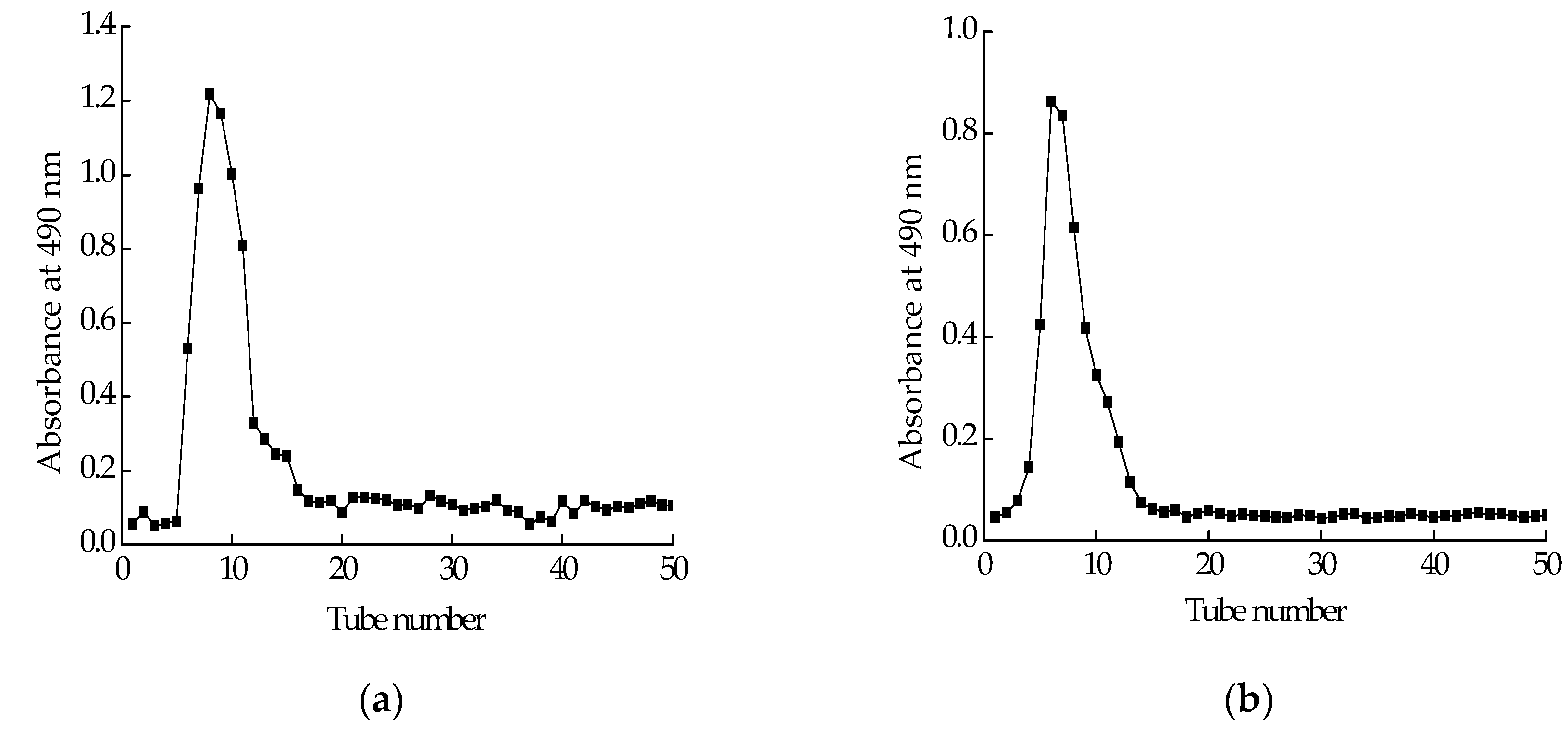
2.2. Isolation and Purification of Polysaccharide
2.3. Molecular Weight and Monosaccharide Composition of CPS-1
2.4. Periodate Oxidation of CSP-1
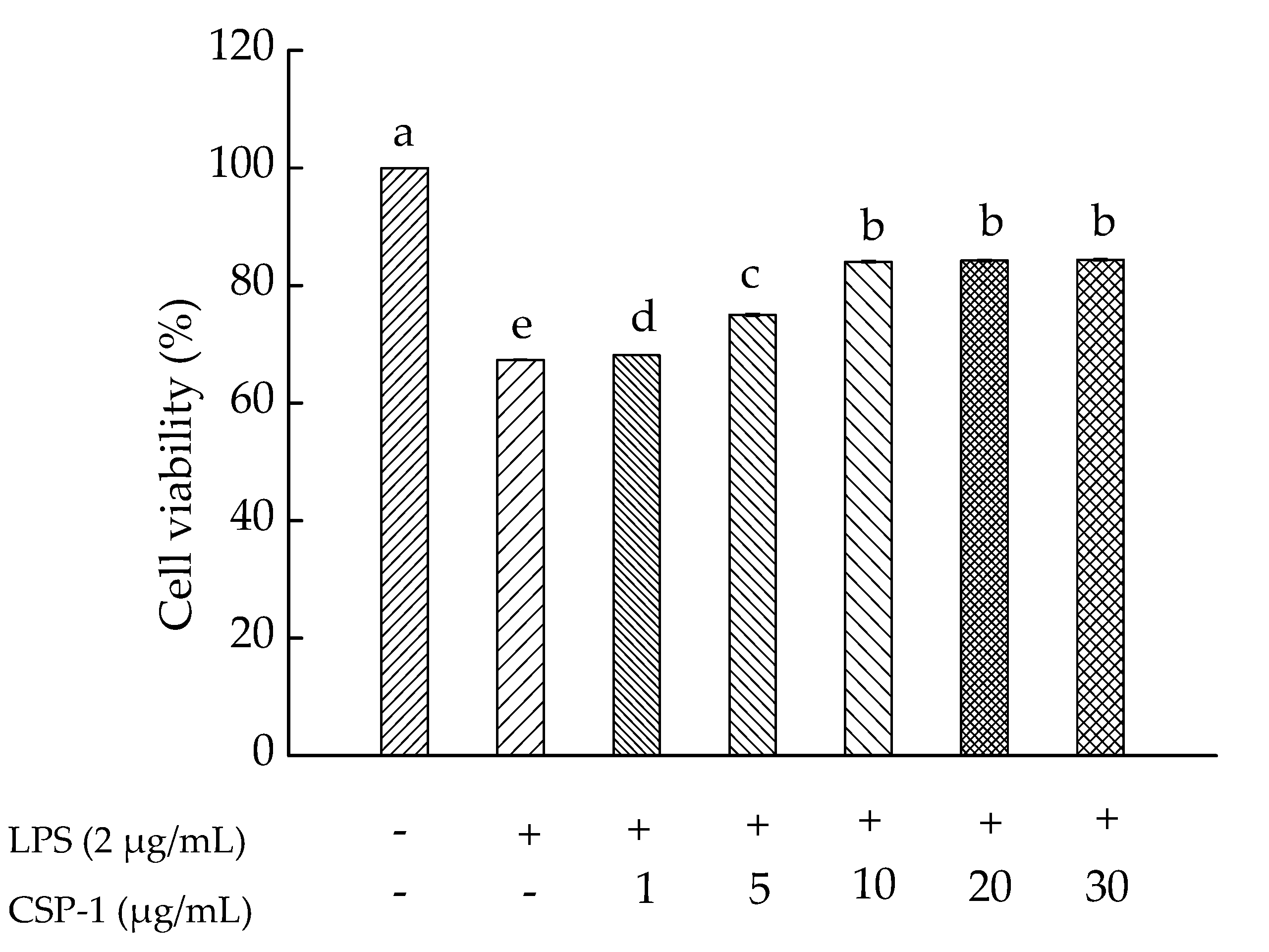
2.5. Effects of CPS-1 on Cell Viability
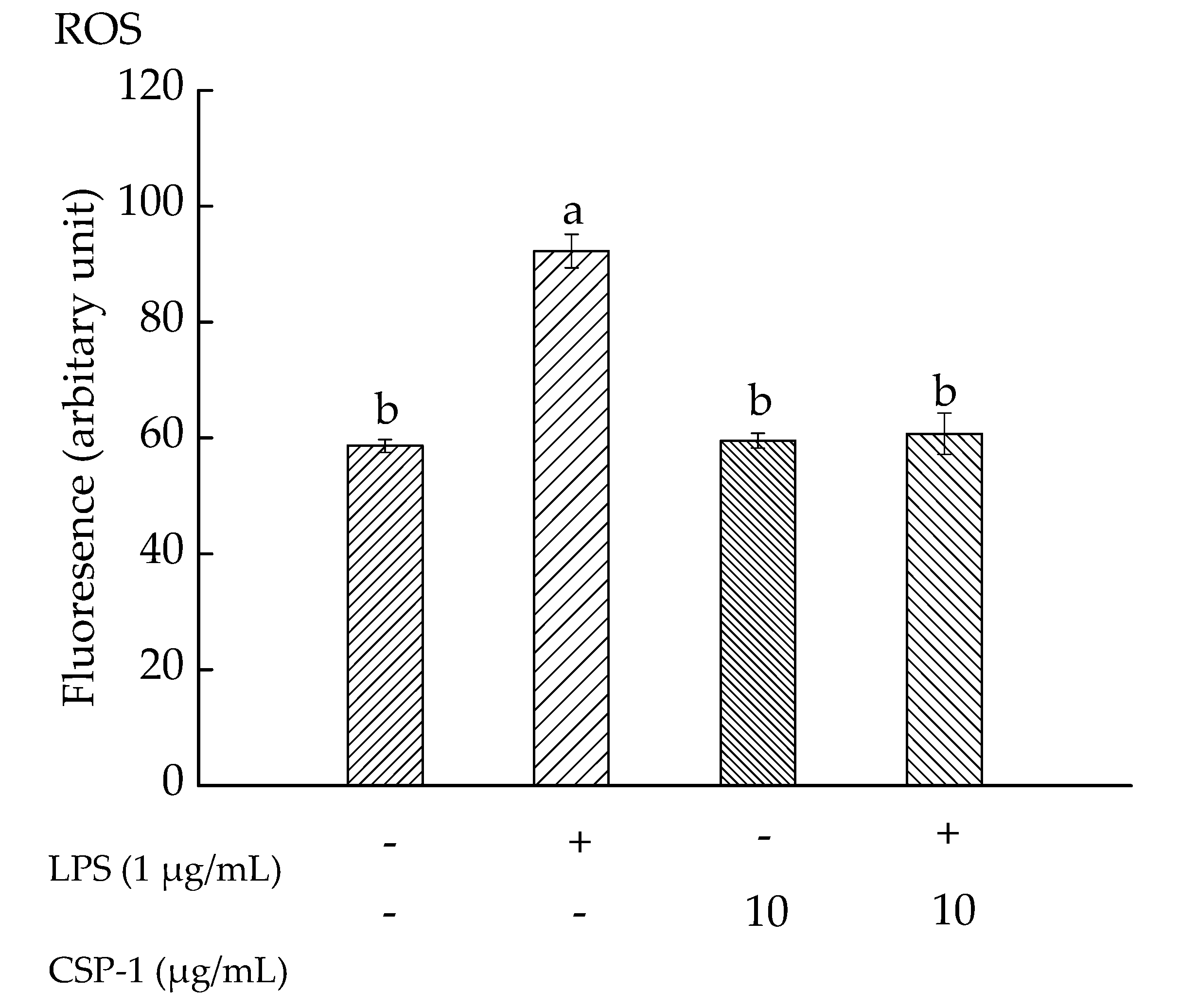
2.6. CPS-1 Inhibited the LPS-Induced ROS Formation
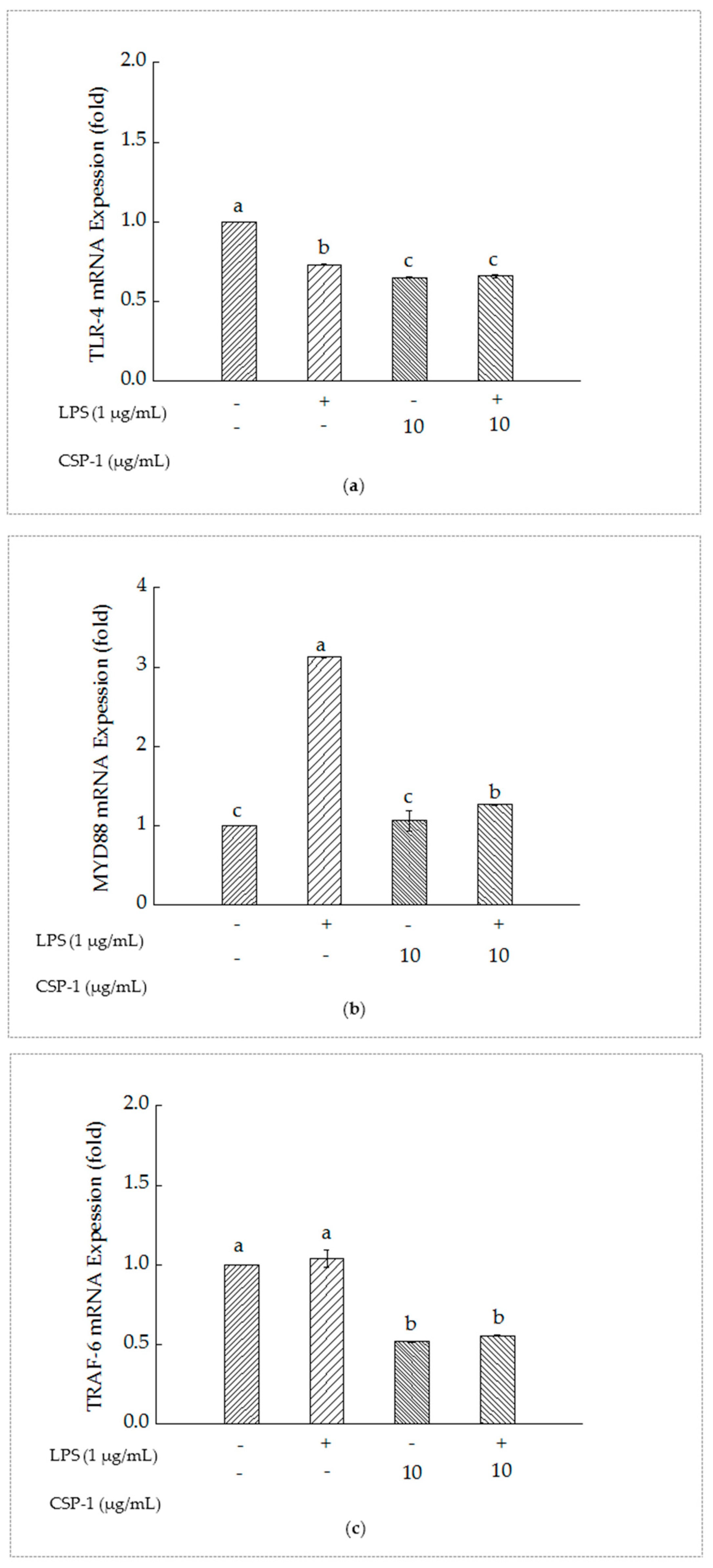
2.7. PSCPL Influenced the TLR-4, MyD88 and TRAF-6 Signal Transduction Pathways
3. Discussion
4. Materials and Methods
4.1. Samples and Materials
4.2. Sample Preparation, Isolation and Purification of the Polysaccharide
4.3. Determination of the Molecular Weight of Polysaccharide
4.4. Analysis of Monosaccharide Composition
4.5. Periodate Oxidation
4.6. Cell Culture
4.7. MTT Assay
4.8. Intracellular ROS Assay
4.9. Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Analysis
4.10. Data Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CSP | Cereus sinensis Polysaccharide |
| DEAE | Diethylaminoethyl cellulose |
| GPC | Gel Permeation Chromatography |
| GS–MS | Gas Chromatography–Mass Spectrometer |
| LPS | Lipopolysaccharide |
| THP-1 cells | Human monocytic cells |
| TLR-4 | Toll-like receptor 4 |
| MyD88 | Myeloid differentiation factor 88 |
| TRAF-6 | Tumour necrosis factor receptor-associated factor-6 |
| PEG | Polyethylene glycol |
| Mn | Number-average molecular weight |
| MW | Weight-average molecular weight |
| MP | Peak molecular weight |
| MZ | Z-average molecular weight |
| MZ+1 | Z+1-average molecular weight |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SD | Standard deviation |
| NF-κB | Nuclear factor kappa B |
| let-7i | Lethal-7i |
| IRAK | Interleukin receptor associated kinase |
| TAK | Transforming growth factor-activated Kinase |
| IKK | Inhibitor of nuclear factor kappa-B kinase |
| IκB | Inhibitor of nuclear factor kappa B |
| TNF-α | Tumor necrosis factor-α |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| EPK | Extracellular signal-regulated kinase |
| p38 | p38 mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| AP | Activator protein |
| RPMI | Roswell Park Memorial Institute |
| NIST | National Insititute of Standards and Technology |
References
- Wen, S.S.; Zhao, X.; Yu, X.L.; Gao, Y.X. The progress of research on Marine Animals Polysaccharides. Chin. J. Mar. Drugs 2009, 4, 46–51. [Google Scholar]
- Liu, J.; Willför, S.; Xu, C.L. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Morabito, M.; Armeli, M.S.; Lo, P.G.; Pagano, M.; Genovese, G. Potential use of polysaccharides from the brown alga Undaria pinnatifida as anticoagulants. Braz. Arch. Biol. Technol. 2015, 58, 798–804. [Google Scholar] [CrossRef]
- Jiao, H.; Shang, X.H.; Dong, Q.; Wang, S.; Liu, X.Y.; Zheng, H.; Lu, X.L. Polysaccharide constituents of three types of sea urchin shells and their anti-inflammatory activities. Mar. Drugs 2015, 13, 5882–5900. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Qian, J.Y.; He, Y.F.; Zheng, J.L.; Lu, Z.M.; Xu, Z.H.; Shi, J.S. Structural and immunological activity characterization of a polysaccharide isolated from Meretrix meretrix Linnaeus. Mar. Drugs 2016, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.Z.; Cao, H.Z.; Wang, F.S. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.X.; Sun, M.L.; Zhao, B.; Yang, X.; Kong, H.X.; Zhang, H.Y.; Sun, L.K.; Kang, J.S. Malignant glioma cells apoptosis induced by sea anemones (Phyllodiscus semoni) venom. J. Apoplexy Nerv. Dis. 2006, 5, 594–596. [Google Scholar]
- Tejucaa, M.; Anderluhb, G.; Serrac, M.D. Sea anemone cytolysins as toxic components of immunotoxins. Toxicon 2009, 54, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Sangappellai, T.; Rajak, R.C.; Diraviam, B. Pharmacological and biomedical properties of sea anemones Paracondactylis indicus, Paracondactylis sinensis, Heteractis magnifica and Stichodactyla haddoni from East coast of India. Asian Pac. J. Trop. Med. 2011, 4, 722–726. [Google Scholar] [CrossRef]
- Shi, W.J.; Qin, S.; Zhao, C.H.; Wu, J.P. Review of sea anemones compound and bioactivity. Mar. Sci. 2013, 37, 122–131. [Google Scholar]
- He, R.J.; Zhao, Y.J.; Zhao, R.N.; Sun, P.L. Study on optimizing homogenate extraction processing and immune activity in vitro of polysaccharide form sea anemone. J. Zhejiang Univ. Technol. 2015, 43, 389–392. [Google Scholar]
- Zheng, S.Z.; Pan, L.J.; Huang, F.L.; Lin, H.Z. The study on the chemical components of polysaccharides from sea anemone Zoanthus Stephensoni. Guangzhou Chem. 1991, 3, 52–56. [Google Scholar]
- Zheng, S.Z.; Pan, L.J.; Huang, F.L.; Gong, L.H. The effects of the polysaccharides from sea anemone Zoanthus Stephenson on perfused isolated rabbit heart tissue and blood pressure of rats. Guangzhou Chem. 1991, 4, 53–57. [Google Scholar]
- Li, Y. Species Composition and Faunastic Characteristics of the Order Actiniaria (Cnidaria: Anthozoa) in Chinese Waters. Ph.D. Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2013. [Google Scholar]
- Shi, W.J.; Zhang, C.H.; Qin, S.; Wu, J.P. Preliminarily isolation and stability analysis of cytolytic toxins from Cereus sinensis Verrill. Chin. J. Mar. Drugs 2013, 32, 14–20. [Google Scholar]
- Zhou, X.F.; Liu, K.H.; Chen, S.S. Nutrients and non-volatile tastecompounds of Cereus sinensis. Sci. Technol. Food Ind. 2016, 37, 345–349. [Google Scholar] [CrossRef]
- Pi, J.H.; Tan, J.; Hu, Z.D. Study on in vitro immune activity of Rosa laevigata polysaccharide. West China J. Pharm. Sci. 2014, 29, 149–151. [Google Scholar]
- Lauriano, E.R.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.; Vreeburg, R.A.; Savelkoul, H.F.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr. Opin. Pharmacol. 2003, 3, 396–403. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish Immunol. 2016, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Wang, H.X.; Liang, L.J.; Lu, M.L.; Gu, J.Y.; He, H.Y. Inhibition of Astragalus polysaccharide in lipopolysccharide-induced cardiomyocyte hypertrophy in rats through the TLR4/NF-κB signal transduction. Chin. Pharmacol. Bull. 2013, 29, 208–212. [Google Scholar]
- Rocch, R.; Kimura, H.; Tzou, S.C.; Suzuki, K.; Rose, N.R.; Pinchera, A.; Ladenson, P.W.; Caturegl, P. Toll-like receptor MyD88 and Fcreceptor pathways of mast cells mediate the thyroid dysfunctions observed during nonthyroidal illness. Proc. Natl. Acad. Sci. USA 2007, 104, 6019–6024. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, Y.; Dai, J.; Medzhitov, R.; Freudenberg, M.A.; Zhang, P.L.; Li, Z. TLR4 up-regulation at protein or gene level is pathogenic for lupus -like autoimmune disease. J. Immunol. 2006, 177, 6880–6888. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kuang, M.; Zhou, H.; Pan, X.C. Progress of Research on TLR4 Signal Pathway and its Negative Regulation. J. Mod. Med. Health 2016, 32, 2821–2824. [Google Scholar]
- Jia, S.J.; Niu, P.P.; Cong, J.Z.; Zhang, B.K.; Zhao, M. TLR4 signaling: A potential therapeutic target in ischemic coronary artery disease. Int. Immunopharmacol. 2014, 23, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Splinter, P.L.; O’ hara, S.P.; LaRusso, N.F. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J. Biol. Chem. 2007, 282, 28929–28938. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Hwang, D. Murine Toll-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NFκB and expression of the inducible cyclooxygenas. J. Biol. Chem. 2000, 275, 34035–34040. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Symmons, M.; Gay, N.J. Toll-like receptor signalling through macromolecular protein complexes. Mol. Immunol. 2015, 63, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, J.K.; Hernandez, A.; Enkhbaatar, P.; Adams, W.L.; Sherwood, E.R. The immunobiology of Toll-like receptor 4 agonists: From endotoxin tolerance to immunoadjuvants. Shock 2013, 40, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Frazão, J.B.; Errante, P.R.; Condino-Neto, A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch. Immunol. Ther. Exp. 2013, 61, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Dërmaku-Sopjani, M.; Abazi, S.; Faggio, C.; Kolgeci, J.; Sopjani, M. AMPK-sensitive cellular transport. J. Biochem. 2014, 155, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Liaw, C.C.; Pan, S.Z.; Yang, H.C.; Ng, L.T. Phellinus linteus polysaccharides and their immunomodulatory properties in human monocytic cells. J. Funct. Food 2013, 5, 679–688. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NFκB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.J.; Wang, J.P.; Zheng, Q.S.; Liu, X.T.; Xue, Y.S.; Li, J.; Zhao, L.Y. The relationship between reactive oxygen species-dependent activation of p38 MAPK and the expression of tumor necrosis factor-α in cultured cardiomyocytes. Chin. J. Cell. Mol. Immunol. 2011, 27, 7–10. [Google Scholar]
| Number | Solid:Liquid Ratio (g/mL) | Time (h) | Temperature (°C) | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 1:90 | 3 | 60 | 21.22 |
| 2 | 1:80 | 2 | 60 | 18.67 |
| 3 | 1:90 | 4 | 70 | 23.20 |
| 4 | 1:70 | 3 | 80 | 21.05 |
| 5 | 1:80 | 4 | 80 | 22.15 |
| 6 | 1:80 | 4 | 60 | 22.30 |
| 7 | 1:70 | 3 | 60 | 20.20 |
| 8 | 1:80 | 3 | 70 | 23.80 |
| 9 | 1:80 | 3 | 70 | 23.76 |
| 10 | 1:80 | 3 | 70 | 23.80 |
| 11 | 1:80 | 3 | 70 | 23.78 |
| 12 | 1:90 | 3 | 80 | 23.34 |
| 13 | 1:70 | 2 | 70 | 20.05 |
| 14 | 1:80 | 2 | 80 | 22.05 |
| 15 | 1:70 | 4 | 70 | 20.98 |
| 16 | 1:80 | 3 | 70 | 24.50 |
| 17 | 1:90 | 2 | 70 | 20.45 |
| Source | Sum of Square | df | Mean Square | F Value | Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 44.85 | 9 | 4.98 | 72.93 | <0.0001 | significant |
| A-Solid:liquid ratio (g/mL) | 4.4 | 1 | 4.4 | 64.32 | <0.0001 | |
| B-Time (h) | 6.86 | 1 | 6.86 | 100.44 | <0.0001 | |
| C-Temperature (°C) | 4.81 | 1 | 4.8 | 70.31 | <0.0001 | |
| AB | 0.83 | 1 | 0.83 | 12.12 | 0.0103 | |
| AC | 0.4 | 1 | 0.4 | 5.9 | 0.0455 | |
| BC | 3.12 | 1 | 3.12 | 45.59 | 0.0003 | |
| A2 | 7.1 | 1 | 7.1 | 103.97 | <0.0001 | |
| B2 | 8.96 | 1 | 8.96 | 131.16 | <0.0001 | |
| C2 | 5.83 | 1 | 5.83 | 85.28 | <0.0001 | |
| Residual | 0.48 | 7 | 0.068 | |||
| Lack of fit | 0.068 | 3 | 0.023 | 0.22 | 0.8767 | not significant |
| Pure error | 0.41 | 4 | 0.1 | |||
| Cor total | 45.33 | 16 |
| Dist Name | Retention Time (min) | Adjusted RT (min) | Mn | MW | MP | MZ | MZ + 1 |
|---|---|---|---|---|---|---|---|
| 28.228 | 28.228 | 2505 | 56,335 | 3937 | 263,586 | 691,260 |
| Name | Retention Time | Type | Peak Width | Peak Area | Starting Time | End Time |
|---|---|---|---|---|---|---|
| l-(−)-Fucose | 12.4 | BB | 0.048 | 13,501,531 | 12.219 | 12.489 |
| d-(+)-Mannose | 15.342 | BV | 0.04 | 1,077,806 | 15.203 | 15.4 |
| d-glucose | 15.488 | VB | 0.042 | 904,727 | 15.4 | 15.562 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhou, X.; Zhang, M.; Yao, Y.; Han, J.; Liu, K. Cereus sinensis Polysaccharide and Its Immunomodulatory Properties in Human Monocytic Cells. Mar. Drugs 2017, 15, 140. https://doi.org/10.3390/md15050140
Wu J, Zhou X, Zhang M, Yao Y, Han J, Liu K. Cereus sinensis Polysaccharide and Its Immunomodulatory Properties in Human Monocytic Cells. Marine Drugs. 2017; 15(5):140. https://doi.org/10.3390/md15050140
Chicago/Turabian StyleWu, Junwen, Xuefei Zhou, Min Zhang, Yun Yao, Juanjuan Han, and Kehai Liu. 2017. "Cereus sinensis Polysaccharide and Its Immunomodulatory Properties in Human Monocytic Cells" Marine Drugs 15, no. 5: 140. https://doi.org/10.3390/md15050140
APA StyleWu, J., Zhou, X., Zhang, M., Yao, Y., Han, J., & Liu, K. (2017). Cereus sinensis Polysaccharide and Its Immunomodulatory Properties in Human Monocytic Cells. Marine Drugs, 15(5), 140. https://doi.org/10.3390/md15050140





