Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense
Abstract
:1. Introduction
2. Infectious Diseases of Marine Bivalve Mollusks
3. Defense Mechanisms in Marine Bivalve Mollusks
4. AMPs and Their Mechanism of Action
5. Marine Bivalve Antimicrobial Peptides
6. Applications of AMPs in Medicine and in Preventing Diseases of Aquatic Animals
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santos, A.; Hauser-Davis, R.A.; Santos, M.J.; De Simone, S.G. Potentially toxic filamentous fungi associated to the economically important Nodipecten nodosus (Linnaeus, 1758) scallop farmed in southeastern Rio De janeiro, Brazil. Mar. Pollut. Bull. 2016, 115, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.E.; Thériault, I.; Mallet, A.L. Infection of cultured eastern oysters Crassostrea virginica by the boring sponge Cliona celata, with emphasis on sponge life history and mitigation strategies. J. Shellfish Res. 2010, 29, 905–915. [Google Scholar] [CrossRef]
- Gagne, N.; Cochennec, N.; Stephenson, M.; McGladdery, S.; Meyer, G.R.; Bower, S.M. First report of a Mikrocytos-like parasite in European oysters Ostrea edulis from Canada after transport and quarantine in France. Dis. Aquat. Organ. 2008, 80, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ford, S.E. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. Evolutionary insights into the origin of innate and adaptive immune systems: Different shades of grey. Asian Pac. J. Allergy Immunol. 2014, 32, 3–15. [Google Scholar] [PubMed]
- Ramilo, A.; Gonzalez, M.; Carballal, M.J.; Darriba, S.; Abollo, E.; Villalba, A. Oyster parasites Bonamia Ostreae and B. exitiosa co-occur in Galicia (NW Spain): Spatial distribution and infection dynamics. Dis. Aquat. Organ. 2014, 110, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, E.; Lanni, L.; Arcangeli, G.; Pepe, T.; Mazzette, R.; Ciccaglioni, G.; Croci, L. Qualitative and quantitative assessment of viral contamination in bivalve molluscs harvested in Italy. Int. J. Food Microbiol. 2014, 184, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Langlade, A.; Chollet, B.; Robert, M.; Ferrand, S.; Omnes, E.; Lerond, S.; Couraleau, Y.; Joly, J.P.; Francois, C.; et al. Can the protozoan parasite Bonamia ostreae infect larvae of flat oysters Ostrea edulis? Vet. Parasitol. 2011, 179, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.M.; Arzul, I.; Pepin, J.F.; Ruano, F.; Friedman, C.S.; Boudry, P.; Renault, T. Detection of ostreid herpesvirus 1 DNA by PCR in bivalve molluscs: A critical review. J. Virol. Methods 2007, 139, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Gao, W.; Wang, C.; Yu, T.; Zhang, T.; Qiu, Z.; Wang, Q.; Huang, J. Identification and characterization of ostreid herpesvirus 1 associated with massive mortalities of Scapharca broughtonii broodstocks in China. Dis. Aquat. Organ. 2016, 118, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.A.; Banfield, W.G.; Kasnic, G., Jr.; Foster, W.S. Oyster herpes-type virus. Science 1972, 178, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Pepin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Carlsson, J.; Reilly, A.O.; Cotter, E.; Culloty, S.C. A previously undescribed Ostreid Herpes Virus 1 (OsHV-1) genotype detected in the Pacific oyster, Crassostrea gigas, in Ireland. Parasitology 2012, 139, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Wang, C.; Xia, J.; Sun, H.; Zhang, S.; Huang, J. Emerging and endemic types of Ostreid herpesvirus 1 were detected in bivalves in China. J. Invertebr. Pathol. 2015, 124, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Park, J.J.; Yu, H.J.; Hur, Y.B.; Arzul, I.; Couraleau, Y.; Park, M.A. Ostreid herpesvirus 1 infection in farmed Pacific oyster larvae Crassostrea gigas (Thunberg) in Korea. J. Fish Dis. 2013, 36, 969–972. [Google Scholar] [PubMed]
- Mortensen, S.; Strand, A.; Bodvin, T.; Alfjorden, A.; Skar, C.K.; Jelmert, A.; Aspan, A.; Saelemyr, L.; Naustvoll, L.J.; Albretsen, J. Summer mortalities and detection of ostreid herpesvirus microvariant in Pacific oyster Crassostrea gigas in Sweden and Norway. Dis. Aquat. Organ. 2016, 117, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Barret, J.; le Gall, P.; Corporeau, C.; Dégremont, L.; Lagarde, F.; Pépin, J.F.; Keck, N. Mass mortalities of Pacific oysters Crassostrea gigas reflect infectious diseases and vary with farming practices in the Mediterranean Thau Iagoon, France. Aquacult. Environ. Interact. 2012, 2, 215–237. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Renault, T.; Fuentes, J.; Villalba, A. Herpesvirus infection in European flat oysters Ostrea edulis obtained from brood stocks of various geographic origins and grown in Galicia (NW Spain). Dis. Aquat. Organ. 2008, 78, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, L.; Elston, R.; Lipovsky, V.P.; Donaldson, J. A new disease of larval Pacific oysters (Crassostrea gigas). J. World Aquacult. Soc. 1978, 9, 603–615. [Google Scholar] [CrossRef]
- Kueh, C.S.; Chan, K.Y. Bacteria in bivalve shellfish with special reference to the oyster. J. Appl. Bacteriol. 1985, 59, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bidault, A.; Richard, G.G.; le Bris, C.; Paillard, C. Development of a Taqman real-time PCR assay for rapid detection and quantification of Vibrio tapetis in extrapallial fluids of clams. PeerJ 2015, 3, e1484. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Labreuche, Y.; Nicolas, J.L. Molecular and phenotypic characterization of Vibrio aestuarianus subsp. francensis subsp. nov., a pathogen of the oyster Crassostrea gigas. Syst. Appl. Microbiol. 2008, 31, 358–365. [Google Scholar] [PubMed]
- Prado, S.; Dubert, J.; da Costa, F.; Martinez-Patino, D.; Barja, J.L. Vibrios in hatchery cultures of the razor clam, Solen marginatus (Pulteney). J. Fish Dis. 2014, 37, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Biel, F.M.; Allen, F.A.; Hase, C.C. Autolysis in Vibrio tubiashii and Vibrio coralliilyticus. Can. J. Microbiol. 2014, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.N.; Bolch, C.J. Genetic diversity of culturable Vibrio in an Australian blue mussel Mytilus galloprovincialis hatchery. Dis. Aquat. Organ. 2015, 116, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, G.; Nakai, T.; Hirata, Y.; Matsubara, D.; Muroga, K. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis. Aquat. Organ. 1998, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Valerio, E.; Chaves, S.; Tenreiro, R. Diversity and impact of prokaryotic toxins on aquatic environments: A review. Toxins (Basel) 2010, 2, 2359–2410. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leon, J.; Villamill, L.; Salger, S.A.; Sallum, R.H.; Remacha-Trivino, A.; Leavitt, D.F.; Gomez-Chiarri, M. Survival of eastern oysters Crassostrea virginica from three lines following experimental challenge with bacterial pathogens. Dis. Aquat. Organ. 2008, 79, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Balboa, S.; Romalde, J.L. Multilocus sequence analysis of Vibrio tapetis, the causative agent of Brown Ring Disease: Description of Vibrio tapetis subsp. britannicus subsp. Nov. Syst. Appl. Microbiol. 2013, 36, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Paillard, C.; Korsnes, K.; Le Chevalier, P.; Le Boulay, C.; Harkestad, L.; Eriksen, A.G.; Willassen, E.; Bergh, O.; Bovo, C.; Skar, C.; et al. Vibrio tapetis-like strain isolated from introduced Manila clams Ruditapes philippinarum showing symptoms of brown ring disease in Norway. Dis. Aquat. Organ. 2008, 81, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Paillard, C. A short-review of brown ring disease, a vibriosis affecting clams, Ruditapes philippinarum and Ruditapes decussatus. Aquat. Living Resour. 2004, 17, 467–475. [Google Scholar] [CrossRef]
- Park, K.I.; Yang, H.S.; Kang, H.S.; Cho, M.; Park, K.J.; Choi, K.S. Isolation and identification of Perkinsus olseni from feces and marine sediment using immunological and molecular techniques. J. Invertebr. Pathol. 2010, 105, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ramilo, A.; Iglesias, D.; Abollo, E.; Gonzalez, M.; Darriba, S.; Villalba, A. Infection of Manila clams Ruditapes philippinarum from Galicia (NW Spain) with a Mikrocytos-like parasite. Dis. Aquat. Organ. 2014, 110, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.; Paillard, C.; Ford, S.E. Pathogenicity of Vibrio tapetis, the etiological agent of brown ring disease in clams. Dis. Aquat. Organ. 2002, 48, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, K.J.; Barber, B.J.; Singer, J.T. Additional evidence that juvenile oyster disease is caused by a member of the Roseobacter group and colonization of nonaffected animals by Stappia stellulata-like strains. Appl. Environ. Microbiol. 2000, 66, 3924–3930. [Google Scholar] [CrossRef] [PubMed]
- Kessner, L.; Spinard, E.; Gomez-Chiarri, M.; Rowley, D.C.; Nelson, D.R. Draft genome sequence of Aliiroseovarius crassostreae CV919–312, the causative agent of Roseovarius oyster disease (formerly juvenile oyster disease). Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.E.; Borrero, F.J. Epizootiology and pathology of juvenile oyster disease in the Eastern oyster, Crassostrea virginica. J. Invertebr. Pathol. 2001, 78, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.E. Roseovarius Oyster Disease (ROD) Caused by Roseovarius crassostreae; ICES Identification Leaflets for Diseases and Parasites of Fish and Shellfish; ICES: Copenhagen, Denmark, 2011. [Google Scholar]
- Friedman, C.S.; Cloney, D.F.; Manzer, D.; Hedrick, R.P. Haplosporidiosis of the Pacific oyster, Crassostrea gigas. J. Invertebr. Pathol. 1991, 58, 367–372. [Google Scholar] [CrossRef]
- Carella, F.; Carrasco, N.; Andree, K.B.; Lacuesta, B.; Furones, D.; De Vico, G. Nocardiosis in Mediterranean bivalves: First detection of Nocardia crassostreae in a new host Mytilus galloprovincialis and in Ostrea edulis from the Gulf of Naples (Italy). J. Invertebr. Pathol. 2013, 114, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.S.; Beattie, J.H.; Elston, R.A.; Hedrick, R.P. Investigation of the relationship between the presence of a Gram-positive bacterial infection and summer mortality of the Pacific oyster, Crassostrea gigas Thunberg. Aquaculture 1991, 94, 1–15. [Google Scholar] [CrossRef]
- Queiroga, F.R.; Marques-Santos, L.F.; De Medeiros, I.A.; Da Silva, P.M. Effects of salinity and temperature on in vitro cell cycle and proliferation of Perkinsus marinus from Brazil. Parasitology 2016, 143, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Robledo, J.A.; Vasta, G.R.; Record, N.R. Protozoan parasites of bivalve molluscs: Literature follows culture. PLoS ONE 2014, 9, e100872. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, R.B.; Meyer, G.R.; Blackbourn, J.; Cochennec-Laureau, N.; Berthe, F.C.; Bower, S.M. Molecular detection of the oyster parasite Mikrocytos mackini, and a preliminary phylogenetic analysis. Dis. Aquat. Organ. 2003, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Lin, X.; Wang, F.; Zhang, Y.; Lv, J.; Wang, C.; Deng, J.; Mei, L.; Wu, S.; Li, H. Detection and characterization of Bonamia ostreae in Ostrea edulis imported to China. Dis. Aquat. Organ. 2013, 106, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.S.; Webb, S.C.; Duncan, J. Bonamia ostreae in the New Zealand oyster Ostrea chilensis: A new host and geographic record for this haplosporidian parasite. Dis. Aquat. Organ. 2016, 118, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hine, P.M.; Cochennec-Laureau, N.; Berthe, F.C. Bonamia exitiosus n. sp. (Haplosporidia) infecting flat oysters Ostrea chilensis in New Zealand. Dis. Aquat. Organ. 2001, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, S.; Arzul, I.; Robert, M.; Berthe, F.C.; Besnard-Cochennec, N.; Crane, M.S. Molecular characterisation of an Australian isolate of Bonamia exitiosa. Dis. Aquat. Organ. 2006, 71, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Dungan, C.F.; Carnegie, R.B.; Hill, K.M.; McCollough, C.B.; Laramore, S.E.; Kelly, C.J.; Stokes, N.A.; Scarpa, J. Diseases of oysters Crassostrea ariakensis and C. virginica reared in ambient waters from the Choptank River, Maryland and the Indian River Lagoon, Florida. Dis. Aquat. Organ. 2012, 101, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.M.; Stokes, N.A.; Webb, S.C.; Hine, P.M.; Kroeck, M.A.; Moore, J.D.; Morley, M.S.; Reece, K.S.; Burreson, E.M.; Carnegie, R.B. Phylogenetics of Bonamia parasites based on small subunit and internal transcribed spacer region ribosomal DNA sequence data. Dis. Aquat. Organ. 2014, 110, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Narcisi, V.; Arzul, I.; Cargini, D.; Mosca, F.; Calzetta, A.; Traversa, D.; Robert, M.; Joly, J.P.; Chollet, B.; Renault, T.; et al. Detection of Bonamia ostreae and B. exitiosa (Haplosporidia) in Ostrea edulis from the Adriatic Sea (Italy). Dis. Aquat. Organ. 2010, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.M.; Carnegie, R.B.; Aloui-Bejaoui, N.; Gharsalli, R.E.; White, D.M.; Stokes, N.A.; Burreson, E.M. Observation of a Bonamia sp. infecting the oyster Ostrea stentina in Tunisia, and a consideration of its phylogenetic affinities. J. Invertebr. Pathol. 2010, 103, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, N.; Villalba, A.; Andree, K.B.; Engelsma, M.Y.; Lacuesta, B.; Ramilo, A.; Gairin, I.; Furones, M.D. Bonamia exitiosa (Haplosporidia) observed infecting the European flat oyster Ostrea edulis cultured on the Spanish Mediterranean coast. J. Invertebr. Pathol. 2012, 110, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Longshaw, M.; Stone, D.M.; Wood, G.; Green, M.J.; White, P. Detection of Bonamia exitiosa (Haplosporidia) in European flat oysters Ostrea edulis cultivated in mainland Britain. Dis. Aquat. Organ. 2013, 106, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.M.; Lopez-Sanmartin, M.; Grade, A.; Navas, J.I.; Ruano, F. Detection of Bonamia exitiosa in the European flat oyster Ostrea edulis in Southern Portugal. J. Fish Dis. 2016, 39, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, R.B.; Burreson, E.M.; Hine, P.M.; Stokes, N.A.; Audemard, C.; Bishop, M.J.; Peterson, C.H. Bonamia perspora n. sp. (Haplosporidia), a parasite of the oyster Ostreola equestris, is the first Bonamia species known to produce spores. J. Eukaryot. Microbiol. 2006, 53, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Armitage, D.V.; Coughlan, J.; Mulcahy, M.F.; Culloty, S.C. Investigating the possible role of benthic macroinvertebrates and zooplankton in the life cycle of the haplosporidian Bonamia ostreae. Exp. Parasitol. 2007, 115, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Cochennec-Laureau, N.; Auffret, M.; Renault, T.; Langlade, A. Changes in circulating and tissue-infiltrating hemocyte parameters of European flat oysters, Ostrea edulis, naturally infected with Bonamia ostreae. J. Invertebr. Pathol. 2003, 83, 23–30. [Google Scholar] [CrossRef]
- Kleeman, S.N.; Adlard, R.D. Molecular detection of Marteilia sydneyi, pathogen of Sydney rock oysters. Dis. Aquat. Organ. 2000, 40, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.; Sereti, V.; Machiels, M.A.; Eding, E.H.; Verreth, J.A. The potential of producing heterotrophic bacteria biomass on aquaculture waste. Water Res. 2006, 40, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Gombac, M.; Kusar, D.; Ocepek, M.; Pogacnik, M.; Arzul, I.; Couraleau, Y.; Jencic, V. Marteiliosis in mussels: A rare disease? J. Fish Dis. 2014, 37, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Aceto, S.; Marrone, R.; Maiolino, P.; De Vico, G. Marteilia refringens infection in cultured and natural beds of mussels (Mytilus galloprovincialis) along the Campanian coast (Tirrenian sea, South of Italy). Bull. Eur. Ass. Fish Pathol. 2010, 30, 189–196. [Google Scholar]
- Tiscar, P.G.; Chagot, D.; Tempesta, M.; Marsilio, F.; Buonavoglia, D. Presenza di Marteilia sp. in mitili (Mytilus galloprovincialis, Lmk) allevati in Puglia. Boll. Soc. Ital. Patol. Ittica 1993, 12, 40–45. [Google Scholar]
- Roubal, F.R.; Masel, J.; Lester, R.J.G. Studies on Marteilia sydneyi, agent of QX disease in the Sydney rock oyster, Saccostrea commercialis, with implications for its life cycle. Aust. J. Mar. Freshw. Res. 1989, 40, 155–167. [Google Scholar] [CrossRef]
- Rubio, A.; Frances, J.; Coad, P.; Stubbs, J.; Guise, K. The onset and termination of the Qx disease window of infection in Sydney rock oyster (Saccostrea glomerata) cultivated in the Hawkesbury River, NSW, Australia. J. Shellfish Res. 2013, 32, 483–496. [Google Scholar] [CrossRef]
- Peruzzi, L.; Gianoglio, B.; Porcellini, G.; Conti, G.; Amore, A.; Coppo, R. Neonatal chronic kidney failure associated with cyclo-oxygenase-2 inhibitors administered during pregnancy. Minerva Urol. Nefrol. 2001, 53, 113–116. [Google Scholar] [PubMed]
- Audemard, C.; Barnaud, A.; Collins, C.M.; Le Roux, F.; Sauriau, P.; Coustau, C.; Blachier, P.; Berthe, F.C. Claire ponds as an experimental model for Marteilia refringens life-cycle studies: New perspectives. J. Exp. Mar. Biol. Ecol. 2001, 257, 87–108. [Google Scholar] [CrossRef]
- Ford, S.E. Dermo Disease of Oysters Caused by Perkinsus marinus; Ford, S.E., Ed.; ICES Identification Leaflets for Diseases and Parasites of Fish and Shellfish; ICES: Copenhagen, Denmark, 2011. [Google Scholar]
- Mackin, J.G.; Owen, H.M.; Collier, A. Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n. sp. in Crassostrea virginica (Gmelin). Science 1950, 111, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Remacha-Trivino, A.; Borsay-Horowitz, D.; Dungan, C.; Gual-Arnau, X.; Gomez-Leon, J.; Villamil, L.; Gomez-Chiarri, M. Numerical quantification of Perkinsus marinus in the American oyster Crassostrea virginica (Gmelin, 1791) (Mollusca: Bivalvia) by modern stereology. J. Parasitol. 2008, 94, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Caceres-Martinez, J.; Madero-Lopez, L.H.; Padilla-Lardizabal, G.; Vasquez-Yeomans, R. Epizootiology of Perkinsus marinus, parasite of the pleasure oyster Crassostrea corteziensis, in the Pacific coast of Mexico. J. Invertebr. Pathol. 2016, 139, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Espinoza, T.L.; Grijalva-Chon, J.M.; Castro-Longoria, R.; Ramos-Paredes, J. Perkinsus marinus in Crassostrea gigas in the Gulf of California. Dis. Aquat. Organ. 2010, 89, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.R.; Vianna, R.T.; Vieira, C.B.; Farias, N.D.; Da Silva, P.M. Parasites infecting the cultured oyster Crassostrea gasar (Adanson, 1757) in Northeast Brazil. Parasitology 2015, 142, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Pagenkopp Lohan, K.M.; Hill-Spanik, K.M.; Torchin, M.E.; Aguirre-Macedo, L.; Fleischer, R.C.; Ruiz, G.M. Richness and distribution of tropical oyster parasites in two oceans. Parasitology 2016, 143, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Paynter, K.T.; Politano, V.; Lane, H.A.; Allen, S.M.; Meritt, D. Growth rates and prevalence of Perkinsus marinus in restored oyster populations in Maryland. J. Shellfish Res. 2010, 29, 309–317. [Google Scholar] [CrossRef]
- Smolowitz, R. A review of current state of knowledge concerning Perkinsus Marinus effects on Crassostrea virginica (Gmelin) (the eastern oyster). Vet. Pathol. 2013, 50, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Corbeil, S.; Morga, B.; Renault, T. Viruses infecting marine molluscs. J. Invertebr. Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leon, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microbiol. 2005, 71, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Froelich, B.A.; Noble, R.T. Vibrio bacteria in raw oysters: Managing risks to human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, R.B.; Hill, K.M.; Stokes, N.A.; Burreson, E.M. The haplosporidian Bonamia exitiosa is present in Australia, but the identity of the parasite described as Bonamia (formerly Mikrocytos) roughleyi is uncertain. J. Invertebr. Pathol. 2014, 115, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Engelsma, M.Y.; Culloty, S.C.; Lynch, S.A.; Arzul, I.; Carnegie, R.B. Bonamia parasites: A rapidly changing perspective on a genus of important mollusc pathogens. Dis. Aquat. Organ. 2014, 110, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Pezzati, E.; Stauder, M.; Grande, C.; Bavestrello, M.; Papetti, A.; Vezzulli, L.; Pruzzo, C. Vibrio cholerae interactions with Mytilus galloprovincialis hemocytes mediated by serum components. Front. Microbiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in invertebrates: Measurement and ecotoxicological relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.G.; Fontanetti, C.S. Hemocitical responses to environmental stress in invertebrates: A review. Environ. Monit. Assess. 2011, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, D.; Ottaviani, E. Cross-talk among immune and neuroendocrine systems in molluscs and other invertebrate models. Horm. Behav. 2017, 88, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bachère, E.; Mialhe, E.; Noël, D.; Boulo, V.; Morvan, A.; Rodriguez, J. Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture 1995, 132, 17–32. [Google Scholar] [CrossRef]
- Evariste, L.; Auffret, M.; Audonnet, S.; Geffard, A.; David, E.; Brousseau, P.; Fournier, M.; Betoulle, S. Functional features of hemocyte subpopulations of the invasive mollusk species Dreissena polymorpha. Fish Shellfish Immunol. 2016, 56, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Boulais, J.; Trost, M.; Landry, C.R.; Dieckmann, R.; Levy, E.D.; Soldati, T.; Michnick, S.W.; Thibault, P.; Desjardins, M. Molecular characterization of the evolution of phagosomes. Mol. Syst. Biol. 2010, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, L.; Qiu, L.; Zhang, H. Bivalve immunity. Adv. Exp. Med. Biol. 2010, 708, 44–65. [Google Scholar] [PubMed]
- Smolowitz, R.M.; Miosky, D.; Reinisch, C.L. Ontogeny of leukemic cells of the soft shell clam. J. Invertebr. Pathol. 1989, 53, 41–51. [Google Scholar] [CrossRef]
- Moore, M.N.; Lowe, D.M. The cytology and cytochemistry of the hemocytes of Mytilus edulis and their responses to experimentally injected carbon particles. J. Invertebr. Pathol. 1977, 29, 18–30. [Google Scholar] [CrossRef]
- Grandiosa, R.; Merien, F.; Pillay, K.; Alfaro, A. Innovative application of classic and newer techniques for the characterization of haemocytes in the New Zealand black-footed abalone (Haliotis iris). Fish Shellfish Immunol. 2016, 48, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rebelo Mde, F.; Figueiredo Ede, S.; Mariante, R.M.; Nobrega, A.; de Barros, C.M.; Allodi, S. New insights from the oyster Crassostrea rhizophorae on bivalve circulating hemocytes. PLoS ONE 2013, 8, e57384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, M.; Chiang, M.W.; Shin, P.K.; Cheung, S.G. Characterization of subpopulations and immune-related parameters of hemocytes in the green-lipped mussel Perna viridis. Fish Shellfish Immunol. 2012, 32, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Renault, T. Monoclonal antibodies to European flat oyster Ostrea edulis hemocytes: Characterization and tissue distribution of granulocytes in adult and developing animals. Dev. Comp. Immunol. 2001, 25, 187–194. [Google Scholar] [CrossRef]
- Lambert, C.; Soudant, P.; Choquet, G.; Paillard, C. Measurement of Crassostrea gigas hemocyte oxidative metabolism by flow cytometry and the inhibiting capacity of pathogenic vibrios. Fish Shellfish Immunol. 2003, 15, 225–240. [Google Scholar] [CrossRef]
- Parisi, M.G.; Li, H.; Jouvet, L.B.; Dyrynda, E.A.; Parrinello, N.; Cammarata, M.; Roch, P. Differential involvement of mussel hemocyte sub-populations in the clearance of bacteria. Fish Shellfish Immunol. 2008, 25, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Renwrantz, L.; Siegmund, E.; Woldmann, M. Variations in hemocyte counts in the mussel, Mytilus edulis: Similar reaction patterns occur in disappearance and return of molluscan hemocytes and vertebrate leukocytes. Comp. Biochem.Physiol. A Mol. Integr. Physiol. 2013, 164, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S.; Ozbay, G.; Kingsley, D.H.; Strauss, M.A. Oyster hemocyte mobilization and increased adhesion activity after β-glucan administration. J. Shellfish Res. 2011, 30, 635–641. [Google Scholar] [CrossRef]
- Taylor, A.M.; Edge, K.J.; Ubrihien, R.P.; Maher, W.A. The freshwater bivalve Corbicula australis as a sentinel species for metal toxicity assessment: An in situ case study integrating chemical and biomarker analyses. Environ. Toxicol. Chem. 2016, 36, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Lanni, L.; Cargini, D.; Narcisi, V.; Bianco, I.; Tiscar, P.G. Variability of the hemocyte parameters of cultivated mussel Mytilus galloprovincialis (Lmk 1819) in Sabaudia (Latina, Italy) coastal lagoon. Mar. Environ. Res. 2013, 92, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Farcy, E.; Burgeot, T.; Haberkorn, H.; Auffret, M.; Lagadic, L.; Allenou, J.P.; Budzinski, H.; Mazzella, N.; Pete, R.; Heydorff, M.; et al. An integrated environmental approach to investigate biomarker fluctuations in the blue mussel Mytilus edulis L. in the Vilaine estuary, France. Environ. Sci. Pollut. Res. Int. 2013, 20, 630–650. [Google Scholar] [CrossRef] [PubMed]
- Hannam, M.L.; Bamber, S.D.; Sundt, R.C.; Galloway, T.S. Immune modulation in the blue mussel Mytilus edulis exposed to north sea produced water. Environ. Pollut. 2009, 157, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Grosvik, B.E.; Borseth, J.F.; Jones, M.B.; Depledge, M.H. Stability of lysosomal and cell membranes in haemocytes of the common mussel (Mytilus edulis): Effect of low temperatures. Mar. Environ. Res. 2000, 50, 325–329. [Google Scholar] [CrossRef]
- Dimitriadis, V.K.; Gougoula, C.; Anestis, A.; Portner, H.O.; Michaelidis, B. Monitoring the biochemical and cellular responses of marine bivalves during thermal stress by using biomarkers. Mar. Environ. Res. 2012, 73, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS ONE 2012, 7, e33820. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, A.; Malham, S.K.; Gelebart, F.; Cueff, A.; Poulet, S.A. Stress-induced immune changes in the oyster Crassostrea gigas. Dev. Comp. Immunol. 2002, 26, 1–9. [Google Scholar] [CrossRef]
- Gagnaire, B.; Frouin, H.; Moreau, K.; Thomas-Guyon, H.; Renault, T. Effects of temperature and salinity on haemocyte activities of the Pacific oyster, Crassostrea gigas (Thunberg). Fish Shellfish Immunol. 2006, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.N.; Burnett, L.E. Reactive oxygen intermediate production by oyster hemocytes exposed to hypoxia. J. Exp. Biol. 1999, 202, 3135–3143. [Google Scholar] [PubMed]
- Buratti, S.; Franzellitti, S.; Poletti, R.; Ceredi, A.; Montanari, G.; Capuzzo, A.; Fabbri, E. Bioaccumulation of algal toxins and changes in physiological parameters in Mediterranean mussels from the North Adriatic Sea (Italy). Environ. Toxicol. 2013, 28, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Hoher, N.; Kohler, A.; Strand, J.; Broeg, K. Effects of various pollutant mixtures on immune responses of the blue mussel (Mytilus edulis) collected at a salinity gradient in danish coastal waters. Mar. Environ. Res. 2012, 75, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Narcisi, V.; Cargini, D.; Calzetta, A.; Tiscar, P.G. Age related properties of the Adriatic clam Chamelea gallina (L. 1758) hemocytes. Fish Shellfish Immunol. 2011, 31, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Tan, T.; Moffit, D.; Deboutteville, J.D.; Barnes, A.C. Gender differences in hemocyte immune parameters of bivalves: The Sydney rock oyster Saccostrea glomerata and the pearl oyster Pinctada fucata. Fish Shellfish Immunol. 2012, 33, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.G.; Abbott, C.A.; Li, X.; Benkendorff, K. Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: An explanation for summer mortality in Pacific oysters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2353–R2362. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.K., Jr.; Smith, E.M.; Barnett, J.A.; Charles, R.; Stefano, G.B. LPS stimulated invertebrate hemocytes: A role for immunoreactive TNF and IL-1. Dev. Comp. Immunol. 1991, 15, 117–122. [Google Scholar] [CrossRef]
- Ottaviani, E.; Franchini, A.; Malagoli, D.; Genedani, S. Immunomodulation by recombinant human interleukin-8 and its signal transduction pathways in invertebrate hemocytes. Cell. Mol. Life Sci. 2000, 57, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Panara, F.; Di Rosa, I.; Fagotti, A.; Simoncelli, F.; Mangiabene, C.; Pipe, R.K.; Pascolini, R. Characterization and immunocytochemical localization of actin and fibronectin in haemocytes of the mussel Mytilus galloprovincialis. Histochem. J. 1996, 28, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, H.; Renwrantz, L. Analysis of the attraction of haemocytes from Mytilus edulis by molecules of bacterial origin. Dev. Comp. Immunol. 1993, 17, 377–387. [Google Scholar] [CrossRef]
- Fawcett, L.B.; Tripp, M.R. Chemotaxis of Mercenaria mercenaria hemocytes to bacteria in vitro. J. Invertebr. Pathol. 1994, 63, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, N.H.; Morimoto, N. Chemotactic activity of hemocytes derived from a brackish-water clam, Corbicula japonica, to Vibrio parahaemolyticus and Escherichia coli strains. J. Vet. Med. Sci. 1992, 54, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Guo, Y.; Sun, R.; Yue, F.; Yi, Q.; Song, L. The broad pattern recognition spectrum of the toll-like receptor in mollusk Zhikong scallop Chlamys farreri. Dev. Comp. Immunol. 2015, 52, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Zhang, G. A Crassostrea gigas toll-like receptor and comparative analysis of TLR pathway in invertebrates. Fish Shellfish Immunol. 2011, 30, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Song, L.; Xu, W.; Ni, D.; Yu, Y. Molecular cloning and expression of a toll receptor gene homologue from Zhikong scallop, Chlamys farreri. Fish Shellfish Immunol. 2007, 22, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.E.; Kraemer, L.; Melzner, F.; Poustka, A.J.; Thieme, S.; Findeisen, U.; Schreiber, S.; Rosenstiel, P. Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis. PLoS ONE 2012, 7, e33091. [Google Scholar] [CrossRef] [PubMed]
- Watters, T.M.; Kenny, E.F.; O’Neill, L.A. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol. Cell Biol. 2007, 85, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. How Toll-like receptors signal: What we know and what we don’t know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, M.; Rosani, U.; Giambelluca, S.; Cammarata, M.; Gerdol, M.; Pallavicini, A.; Venier, P.; Roch, P. Toll signal transduction pathway in bivalves: Complete CDS of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis. Dev. Comp. Immunol. 2014, 45, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Zhou, Z.; Qiu, L.; Wang, L.; Zhang, H.; Gao, Y.; Wang, X.; Zhang, L.; Zhao, J.; et al. A primitive Toll-like receptor signaling pathway in mollusk Zhikong scallop Chlamys farreri. Dev. Comp. Immunol. 2011, 35, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Balseiro, P.; Planas, J.V.; Fuste, B.; Beltran, S.; Novoa, B.; Figueras, A. Transcriptomics of in vitro immune-stimulated hemocytes from the Manila Clam Ruditapes philippinarum using high-throughput sequencing. PLoS ONE 2012, 7, e35009. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Xiang, Z.; Wang, F.; Zhang, Y.; Li, J.; Zhang, Y.; Xiao, S.; Yu, Z. Identification and function of an evolutionarily conserved signaling intermediate in toll pathways (ECSIT) from Crassostrea hongkongensis. Dev. Comp. Immunol. 2015, 53, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Betti, M.; Ciacci, C.; Lorusso, L.C.; Gallo, G.; Pruzzo, C. Interactions between Mytilus haemocytes and different strains of Escherichia coli and Vibrio cholerae O1 El Tor: Role of kinase-mediated signalling. Cell. Microbiol. 2005, 7, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Citterio, B.; Betti, M.; Canonico, B.; Roch, P.; Canesi, L. Functional differential immune responses of Mytilus galloprovincialis to bacterial challenge. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, R.; Zhang, T.; Zhang, R.; Song, X.; Wang, L.; Song, L. A novel phagocytic receptor (CgNimC) from Pacific oyster Crassostrea gigas with lipopolysaccharide and Gram-negative bacteria binding activity. Fish Shellfish Immunol. 2015, 43, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Song, L.; Wu, L.; Chang, Y.; Yu, Y.; Qiu, L.; Wang, L. Molecular cloning and mRNA expression of peptidoglycan recognition protein (PGRP) gene in bay scallop (Argopecten irradians, Lamarck 1819). Dev. Comp. Immunol. 2007, 31, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, J.; Liu, X.; Yang, D.; Xu, J.; Fang, J.; Wang, W.; Yang, J. Identification and transcriptional analysis of two types of lectins (SgCTL-1 and SgGal-1) from mollusk Solen grandis. Fish Shellfish Immunol. 2012, 33, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Gallo, G.; Gavioli, M.; Pruzzo, C. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 2002, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Carballal, M.J.; Lopez, C.; Azevedo, C.; Villalba, A. Enzymes involved in defense functions of hemocytes of mussel Mytilus galloprovincialis. J. Invertebr. Pathol. 1997, 70, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Matozzo, V.; Marin, M.G.; Ballarin, L. Haemocytes of the clam Tapes philippinarum (Adams & Reeve, 1850): Morphofunctional characterisation. Fish Shellfish Immunol. 2000, 10, 677–693. [Google Scholar] [PubMed]
- Matozzo, V.; Rova, G.; Marin, M.G. Haemocytes of the cockle Cerastoderma glaucum: Morphological characterisation and involvement in immune responses. Fish Shellfish Immunol. 2007, 23, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Wootton, E.C.; Dyrynda, E.A.; Ratcliffe, N.A. Bivalve immunity: Comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua). Fish Shellfish Immunol. 2003, 15, 195–210. [Google Scholar] [CrossRef]
- Pampanin, D.M.; Marin, M.G.; Ballarin, L. Morphological and cytoenzymatic characterization of haemocytes of the Venus Clam Chamelea gallina. Dis. Aquat. Organ. 2002, 49, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S. Reactive oxygen species and antimicrobial defenses of invertebrates: A bivalve model. Adv. Exp. Med. Biol. 2001, 484, 131–139. [Google Scholar] [PubMed]
- Adema, C.M.; van Deutekom-Mulder, E.C.; van der Knaap, W.P.; Sminia, T. NADPH-oxidase activity: The probable source of reactive oxygen intermediate generation in hemocytes of the gastropod Lymnaea stagnalis. J. Leukoc. Biol. 1993, 54, 379–383. [Google Scholar] [PubMed]
- Connors, V.A.; Lodes, M.J.; Yoshino, T.P. Identification of a Schistosoma mansoni sporocyst excretory-secretory antioxidant molecule and its effect on superoxide production by Biomphalaria glabrata hemocytes. J. Invertebr. Pathol. 1991, 58, 387–395. [Google Scholar] [CrossRef]
- Ordas, M.C.; Novoa, B.; Figueras, A. Modulation of the chemiluminescence response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes. Fish Shellfish Immunol. 2000, 10, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Goedken, M.; De Guise, S. Flow cytometry as a tool to quantify oyster defense mechanisms. Fish Shellfish Immunol. 2004, 16, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Bugge, D.M.; Hegaret, H.; Wikfors, G.H.; Allam, B. Oxidative burst in hard clam (Mercenaria mercenaria) haemocytes. Fish Shellfish Immunol. 2007, 23, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Winston, G.W.; Moore, M.N.; Kirchin, M.A.; Soverchia, C. Production of reactive oxygen species by hemocytes from the marine mussel, Mytilus edulis: Ysosomal localization and effect of xenobiotics. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 113, 221–229. [Google Scholar] [CrossRef]
- Donaghy, L.; Kraffe, E.; Le Goic, N.; Lambert, C.; Volety, A.K.; Soudant, P. Reactive oxygen species in unstimulated hemocytes of the Pacific oyster Crassostrea gigas: A mitochondrial involvement. PLoS ONE 2012, 7, e46594. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Li, D.; Gao, Y.; Cao, X. The roles of lysosomes in inflammation and autoimmune diseases. Int. Rev. Immunol. 2015, 34, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.R.; Spurmanis, A.; Siah, A.; Araya, M.T.; Kulka, M.; Berthe, F.C.; Johnson, G.R.; Greenwood, S.J. Changes induced by two strains of Vibrio splendidus in haemocyte subpopulations of Mya Arenaria, detected by flow cytometry with LysoTracker. Dis. Aquat. Organ. 2009, 86, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.; Ashton-Alcox, K.A.; Ford, S.E. Flow cytometric comparison of haemocytes from three species of bivalve molluscs. Fish Shellfish Immunol. 2002, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, E.; Prado-Alvarez, M.; Novoa, B.; Figueras, A.; Rosales, C. Immune responses of mussel hemocyte subpopulations are differentially regulated by enzymes of the PI 3-K, PKC, and ERK kinase families. Dev. Comp. Immunol. 2008, 32, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Tunkijjanukij, S.; Giaever, H.; Chin, C.C.; Olafsen, J.A. Sialic acid in hemolymph and affinity purified lectins from two marine bivalves. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 119, 705–713. [Google Scholar] [CrossRef]
- Olafsen, J.A. Bacterial antigen priming of marine fish larvae. Adv. Exp. Med. Biol. 1995, 371A, 349–352. [Google Scholar] [PubMed]
- Yang, J.; Huang, M.; Zhang, H.; Wang, L.; Wang, H.; Wang, L.; Qiu, L.; Song, L. CfLec-3 from scallop: An entrance to non-self recognition mechanism of invertebrate C-type lectin. Sci. Rep. 2015, 5, 10068. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Chen, L.; Zhao, J.; Wang, C. Molecular cloning and expression of a C-type lectin gene from Venerupis philippinarum. Mol. Biol. Rep. 2014, 41, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, L.; Yang, J.; Zhang, H.; Wang, L.; Song, L. A four-CRD C-type lectin from Chlamys farreri mediating nonself-recognition with broader spectrum and opsonization. Dev. Comp. Immunol. 2013, 39, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [PubMed]
- Ahmed, H.; Vasta, G.R. Galectins: Conservation of functionally and structurally relevant amino acid residues defines two types of carbohydrate recognition domains. Glycobiology 1994, 4, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Bianchet, M.A.; Amzel, L.M.; Hirabayashi, J.; Kasai, K.; Giga-Hama, Y.; Tohda, H.; Vasta, G.R. Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: Binding to blood group precursor oligosaccharides (type 1, type 2, Talpha, and Tbeta) and gangliosides. Glycobiology 2002, 12, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Bianchet, M.A.; Ahmed, H.; Vasta, G.R.; Amzel, L.M. Soluble beta-galactosyl-binding lectin (galectin) from toad ovary: Crystallographic studies of two protein-sugar complexes. Proteins 2000, 40, 378–388. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.M.; Cho, S.K.; Choi, K.S.; Cho, M. Noble tandem-repeat galectin of Manila Clam Ruditapes philippinarum is induced upon infection with the protozoan parasite Perkinsus olseni. Dev. Comp. Immunol. 2008, 32, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, H.; Wang, L.; Zhao, J.; Mu, C.; Song, L.; Qiu, L.; Liu, X. A galectin with quadruple-domain from bay scallop Argopecten irradians is involved in innate immune response. Dev. Comp. Immunol. 2011, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ghosh, A.; Amin, M.N.; Giomarelli, B.; Shridhar, S.; Banerjee, A.; Fernandez-Robledo, J.A.; Bianchet, M.A.; Wang, L.X.; Wilson, I.B.; et al. The galectin CvGal1 from the eastern oyster (Crassostrea virginica) binds to blood group a oligosaccharides on the hemocyte surface. J. Biol. Chem. 2013, 288, 24394–24409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, H.; Zhang, D.; Zhang, H.; Wang, L.; Sun, J.; Song, L. A C1q domain containing protein from Crassostrea gigas serves as pattern recognition receptor and opsonin with high binding affinity to LPS. Fish Shellfish Immunol. 2015, 45, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Manfrin, C.; De Moro, G.; Figueras, A.; Novoa, B.; Venier, P.; Pallavicini, A. The C1q domain containing proteins of the Mediterranean mussel Mytilus galloprovincialis: A widespread and diverse family of immune-related molecules. Dev. Comp. Immunol. 2011, 35, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhang, H.; Wang, L.; Zhou, Z.; Yang, J.; Zhang, Y.; Qiu, L.; Wang, L.; Song, L. AiC1qDC-1, a novel gC1q-domain-containing protein from bay scallop Argopecten irradians with fungi agglutinating activity. Dev. Comp. Immunol. 2010, 34, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, S.; Zhao, J.; Su, X.; Li, T. Cloning and characterization of a sialic acid binding lectins (SABL) from Manila Clam Venerupis Philippinarum. Fish Shellfish Immunol. 2011, 30, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Venier, P.; Pallavicini, A. The genome of the Pacific oyster Crassostrea gigas brings new insights on the massive expansion of the C1q gene family in Bivalvia. Dev. Comp. Immunol. 2015, 49, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Zhang, D.; Jiang, Q.; Sun, R.; Wang, H.; Zhang, H.; Song, L. A novel multi-domain C1qDC protein from Zhikong scallop Chlamys farreri provides new insights into the function of invertebrate C1qDC proteins. Dev. Comp. Immunol. 2015, 52, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xie, J.; Li, J.; Luo, M.; Ye, S.; Wu, X. Identification of expressed genes in cDNA library of hemocytes from the RLO-challenged oyster, Crassostrea ariakensis gould with special functional implication of three complement-related fragments (CaC1q1, CaC1q2 and CaC3). Fish Shellfish Immunol. 2012, 32, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhou, C.; Zhu, L.; Huang, Y.; Yan, T.; Fang, J.; Zhu, W. Identification and expression analysis on bactericidal permeability-increasing protein (BPI)/lipopolysaccharide-binding protein (LBP) of ark shell, Scapharca broughtonii. Fish Shellfish Immunol. 2013, 35, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Li, X.; Fu, D.; Chen, J.; Yu, Z. The second bactericidal permeability increasing protein (BPI) and its revelation of the gene duplication in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2011, 30, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Gueguen, Y.; Destoumieux-Garzon, D.; Romestand, B.; Fievet, J.; Pugniere, M.; Roquet, F.; Escoubas, J.M.; Vandenbulcke, F.; Levy, O.; et al. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc. Natl. Acad. Sci. USA 2007, 104, 17759–17764. [Google Scholar] [CrossRef] [PubMed]
- Balbi, T.; Fabbri, R.; Cortese, K.; Smerilli, A.; Ciacci, C.; Grande, C.; Vezzulli, L.; Pruzzo, C.; Canesi, L. Interactions between Mytilus galloprovincialis hemocytes and the bivalve pathogens Vibrio aestuarianus 01/032 and Vibrio splendidus LGP32. Fish Shellfish Immunol. 2013, 35, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, J.; Wu, H.; Wang, Q. Metabolomic analysis revealed the differential responses in two pedigrees of clam Ruditapes philippinarum towards Vibrio harveyi challenge. Fish Shellfish Immunol. 2013, 35, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Shi, X.; Zhang, H.; Gao, Y.; Wang, M.; Kong, P.; Qiu, L.; Song, L. The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri. Fish Shellfish Immunol. 2011, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Morga, B.; Renault, T.; Faury, N.; Chollet, B.; Arzul, I. Cellular and molecular responses of haemocytes from Ostrea edulis during in vitro infection by the parasite Bonamia ostreae. Int. J. Parasitol. 2011, 41, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.G.; Schey, K.L.; Volety, A.K.; Chu, F.L.; La Peyre, J.F. Purification and characterization of lysozyme from plasma of the eastern oyster (Crassostrea virginica). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.; Pales Espinosa, E.; Tanguy, A.; Jeffroy, F.; Le Bris, C.; Paillard, C. Transcriptional changes in Manila clam (Ruditapes philippinarum) in response to Brown Ring Disease. Fish Shellfish Immunol. 2014, 41, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Vance, K.; Gomez-Chiarri, M. Protease activity in the plasma of American oysters, Crassostrea virginica, experimentally infected with the protozoan parasite Perkinsus marinus. J. Parasitol. 2003, 89, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Jin, K.; Wang, L.; Feng, B.; Li, J. Molecular characterization and expression analysis of four cathepsin L genes in the razor clam, Sinonovacula constricta. Fish Shellfish Immunol. 2013, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.; Pales Espinosa, E. Bivalve immunity and response to infections: Are we looking at the right place? Fish Shellfish Immunol. 2016, 53, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- Charles, F.; Grémare, A.; Amouroux, J.-M.; Cahet, G. Filtration of the enteric bacteria Escherichia coli by two filter-feeding bivalves, Venus verrucosa and Mytilus galloprovincialis. Mar. Biol. 1992, 113, 125–131. [Google Scholar] [CrossRef]
- McHenery, J.G. Uptake and processing of cultured microorganisms by bivalves. J. Exp. Mar. Biol. Ecol. 1985, 90, 145–163. [Google Scholar] [CrossRef]
- Otero-Gonzalez, A.J.; Magalhaes, B.S.; Garcia-Villarino, M.; Lopez-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defenses. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Radek, K.; Gallo, R. Antimicrobial peptides: Natural effectors of the innate immune system. Semin. Immunopathol. 2007, 29, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Rozek, A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 2002, 206, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rozek, A.; Hancock, R.E. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 2001, 276, 35714–35722. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, G.; Lecar, H. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 1977, 10, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hall, K.N.; Aguilar, M.I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; de Vries, A.H.; Tieleman, D.P. Lipids on the move: Simulations of membrane pores, domains, stalks and curves. Biochim. Biophys. Acta 2009, 1788, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Galdiero, M.; Galdiero, S. Membranotropic cell penetrating peptides: The outstanding journey. Int. J. Mol. Sci. 2015, 16, 25323–25337. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Vitiello, M.; Falanga, A.; Cantisani, M.; Incoronato, N.; Galdiero, M. Intracellular delivery: Exploiting viral membranotropic peptides. Curr. Drug Metab. 2012, 13, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, N.; Budagavi, D.P.; Yadav, B.K.; Chugh, A. Membrane-active peptides from marine organisms—Antimicrobials, cell-penetrating peptides and peptide toxins: Applications and prospects. Probiotics Antimicrob. Proteins 2015, 7, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals (Basel) 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine antimicrobial peptides: Nature provides templates for the design of novel compounds against pathogenic bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef] [PubMed]
- Hubert, F.; Noel, T.; Roch, P. A member of the arthropod defensin family from edible Mediterranean mussels (Mytilus galloprovincialis). Eur. J. Biochem. 1996, 240, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Charlet, M.; Chernysh, S.; Philippe, H.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Innate immunity. Isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J. Biol. Chem. 1996, 271, 21808–21813. [Google Scholar] [PubMed]
- Mitta, G.; Hubert, F.; Noel, T.; Roch, P. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis. Eur. J. Biochem. 1999, 265, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Vandenbulcke, F.; Hubert, F.; Roch, P. Mussel defensins are synthesised and processed in granulocytes then released into the plasma after bacterial challenge. J. Cell Sci. 1999, 112, 4233–4242. [Google Scholar] [PubMed]
- Yang, Y.S.; Mitta, G.; Chavanieu, A.; Calas, B.; Sanchez, J.F.; Roch, P.; Aumelas, A. Solution structure and activity of the synthetic four-disulfide bond Mediterranean mussel defensin (MGD-1). Biochemistry 2000, 39, 14436–14447. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Unifying themes in host defense effector polypeptides. Nat. Rev. Microbiol. 2007, 5, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Galdiero, S.; Cantisani, M.; Di Noto, R.; Vitiello, M.; Galdiero, M.; Naclerio, G.; Cassiman, J.J.; Pedone, C.; Castaldo, G.; et al. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob. Agents Chemother. 2010, 54, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Galdiero, S.; Nigro, E.; Del Vecchio, L.; Di Noto, R.; Cantisani, M.; Colavita, I.; Galdiero, M.; Cassiman, J.J.; Daniele, A.; et al. Chimeric beta-defensin analogs, including the novel 3NI analog, display salt-resistant antimicrobial activity and lack toxicity in human epithelial cell lines. Antimicrob. Agents Chemother. 2013, 57, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Colavita, I.; Sarnataro, D.; Scudiero, O.; Zambrano, G.; Granata, V.; Daniele, A.; Carotenuto, A.; Galdiero, S.; Folliero, V.; et al. An ancestral host defense peptide within human beta-defensin 3 recapitulates the antibacterial and antiviral activity of the full-length molecule. Sci. Rep. 2015, 5, 18450. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Nigro, E.; Cantisani, M.; Colavita, I.; Leone, M.; Mercurio, F.A.; Galdiero, M.; Pessi, A.; Daniele, A.; Salvatore, F.; et al. Design and activity of a cyclic mini-beta-defensin analog: A novel antimicrobial tool. Int. J. Nanomed. 2015, 10, 6523–6539. [Google Scholar] [PubMed]
- Romestand, B.; Molina, F.; Richard, V.; Roch, P.; Granier, C. Key role of the loop connecting the two beta strands of mussel defensin in its antimicrobial activity. Eur. J. Biochem. 2003, 270, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Vandenbulcke, F.; Hubert, F.; Salzet, M.; Roch, P. Involvement of mytilins in mussel antimicrobial defense. J. Biol. Chem. 2000, 275, 12954–12962. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Hubert, F.; Dyrynda, E.A.; Boudry, P.; Roch, P. Mytilin Band MGD2, two antimicrobial peptides of marine mussels: Gene structure and expression analysis. Dev. Comp. Immunol. 2000, 24, 381–393. [Google Scholar] [CrossRef]
- Balseiro, P.; Falco, A.; Romero, A.; Dios, S.; Martinez-Lopez, A.; Figueras, A.; Estepa, A.; Novoa, B. Mytilus galloprovincialis Myticin C: A chemotactic molecule with antiviral activity and immunoregulatory properties. PLoS ONE 2011, 6, e23140. [Google Scholar] [CrossRef] [PubMed]
- Domeneghetti, S.; Franzoi, M.; Damiano, N.; Norante, R.; El Halfawy, N.M.; Mammi, S.; Marin, O.; Bellanda, M.; Venier, P. Structural and antimicrobial features of peptides related to Myticin C, a special defense molecule from the Mediterranean mussel Mytilus galloprovincialis. J. Agric. Food Chem. 2015, 63, 9251–9259. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Romero, A.; Alvarez, A.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of Myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wang, X.C.; Liu, H.H.; Fan, M.H.; Sun, J.J.; Shen, W. Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immunol. 2013, 34, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.L.; Huang, W.; Zhou, S.Q.; Wang, X.C.; Liu, H.H.; Fan, M.H.; Wang, R.X.; Gao, P.; Liao, Z. Characterization of a novel antimicrobial peptide with chitin-biding domain from Mytilus coruscus. Fish Shellfish Immunol. 2014, 41, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; De Moro, G.; Manfrin, C.; Venier, P.; Pallavicini, A. Big defensins and mytimacins, new AMP families of the Mediterranean mussel Mytilus galloprovincialis. Dev. Comp. Immunol. 2012, 36, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, C.; Chen, A.; Li, L.; Su, X.; Li, T. Molecular characterization of a novel big defensin from clam Venerupis philippinarum. PLoS ONE 2010, 5, e13480. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Hua, L.; Jian-Min, Z.; Lin-Sheng, S. Molecular characterization and expression of a novel big defensin (Sb-BDef1) from ark shell, Scapharca broughtonii. Fish Shellfish Immunol. 2012, 33, 1167–1173. [Google Scholar]
- Gestal, C.; Costa, M.; Figueras, A.; Novoa, B. Analysis of differentially expressed genes in response to bacterial stimulation in hemocytes of the carpet-shell clam Ruditapes decussatus: Identification of new antimicrobial peptides. Gene 2007, 406, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Crawford, J.M.; Stone, K.L.; Noga, E.J. Purification of a novel arthropod defensin from the American oyster, Crassostrea virginica. Biochem. Biophys. Res. Commun. 2005, 338, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, Y.; Herpin, A.; Aumelas, A.; Garnier, J.; Fievet, J.; Escoubas, J.M.; Bulet, P.; Gonzalez, M.; Lelong, C.; Favrel, P.; et al. Characterization of a defensin from the oyster Crassostrea gigas. Recombinant production, folding, solution structure, antimicrobial activities, and gene expression. J. Biol. Chem. 2006, 281, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Gueguen, Y.; Desserre, G.; de Lorgeril, J.; Romestand, B.; Bachere, E. Molecular characterization of two isoforms of defensin from hemocytes of the oyster Crassostrea gigas. Dev. Comp. Immunol. 2007, 31, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.-M.; Song, L.-S. A review of advances in research on marine molluscan antimicrobial peptides and their potential application in aquaculture. Molluscan Res. 2009, 29, 17–26. [Google Scholar]
- Gueguen, Y.; Bernard, R.; Julie, F.; Paulina, S.; Delphine, D.G.; Franck, V.; Philippe, B.; Evelyne, B. Oyster hemocytes express a proline-rich peptide displaying synergistic antimicrobial activity with a defensin. Mol. Immunol. 2009, 46, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, P.; de Lorgeril, J.; Gueguen, Y.; Destoumieux-Garzon, D.; Bachere, E. Expression, tissue localization and synergy of antimicrobial peptides and proteins in the immune response of the oyster Crassostrea gigas. Dev. Comp. Immunol. 2012, 37, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, L.; Li, C.; Ni, D.; Wu, L.; Zhu, L.; Wang, H.; Xu, W. Molecular cloning, expression of a big defensin gene from bay scallop Argopecten irradians and the antimicrobial activity of its recombinant protein. Mol. Immunol. 2007, 44, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Arenas, G.; Guzman, F.; Cardenas, C.; Mercado, L.; Marshall, S.H. A novel antifungal peptide designed from the primary structure of a natural antimicrobial peptide purified from Argopecten purpuratus hemocytes. Peptides 2009, 30, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Berisio, R.; Grieco, P.; Morelli, G.; Galdiero, M. Antimicrobial peptides as an opportunity against bacterial diseases. Curr. Med. Chem. 2015, 22, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Wain, L.V.; Shrine, N.; Miller, S.; Jackson, V.E.; Ntalla, I.; Soler Artigas, M.; Billington, C.K.; Kheirallah, A.K.; Allen, R.; Cook, J.P.; et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): A genetic association study in UK Biobank. Lancet Respir. Med. 2015, 3, 769–781. [Google Scholar] [CrossRef]
- Barton, M.D.; Ndi, O.L. Can we feel it in our waters? Antimicrobials in aquaculture. Med. J. Aust. 2012, 197, 487. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Agerso, Y.; Gerner-Smidt, P.; Madsen, M.; Jensen, L.B. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in denmark. Diagn. Microbiol. Infect. Dis. 2000, 37, 127–137. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, A.H.; Tomova, A.; Lopez, A.; Maldonado, M.A.; Henriquez, L.A.; Ivanova, L.; Moy, F.; Godfrey, H.P.; Cabello, F.C. Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS ONE 2012, 7, e42724. [Google Scholar] [CrossRef] [PubMed]
- Avan, I.; Hall, C.D.; Katritzky, A.R. Peptidomimetics via modifications of amino acids and peptide bonds. Chem. Soc. Rev. 2014, 43, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Qvit, N.; Rubin, S.J.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.R. Peptidomimetic therapeutics: Scientific approaches and opportunities. Drug Discov. Today 2017, 22, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Danial, M.; van Dulmen, T.H.; Aleksandrowicz, J.; Potgens, A.J.; Klok, H.A. Site-specific PEGylation of HR2 peptides: Effects of PEG conjugation position and chain length on HIV-1 membrane fusion inhibition and proteolytic degradation. Bioconjug. Chem. 2012, 23, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Oren, Z.; Pag, U.; Sahl, H.G.; Shai, Y. The consequence of sequence alteration of an amphipathic alpha-helical antimicrobial peptide and its diastereomers. J. Biol. Chem. 2002, 277, 33913–33921. [Google Scholar] [CrossRef] [PubMed]
- Mojsoska, B.; Jenssen, H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals (Basel) 2015, 8, 366–415. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, M.; Finamore, E.; Mignogna, E.; Falanga, A.; Nicoletti, G.F.; Pedone, C.; Morelli, G.; Leone, M.; Galdiero, M.; Galdiero, S. Structural insights into and activity analysis of the antimicrobial peptide myxinidin. Antimicrob. Agents Chemother. 2014, 58, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, M.; Leone, M.; Mignogna, E.; Kampanaraki, K.; Falanga, A.; Morelli, G.; Galdiero, M.; Galdiero, S. Structure-activity relations of myxinidin, an antibacterial peptide derived from the epidermal mucus of hagfish. Antimicrob. Agents Chemother. 2013, 57, 5665–5673. [Google Scholar] [CrossRef] [PubMed]
- Kustanovich, I.; Shalev, D.E.; Mikhlin, M.; Gaidukov, L.; Mor, A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 2002, 277, 16941–16951. [Google Scholar] [CrossRef] [PubMed]
- Zelezetsky, I.; Pag, U.; Sahl, H.G.; Tossi, A. Tuning the biological properties of amphipathic alpha-helical antimicrobial peptides: Rational use of minimal amino acid substitutions. Peptides 2005, 26, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Post-translational modifications of natural antimicrobial peptides and strategies for peptide engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Fedders, H.; Michalek, M.; Grotzinger, J.; Leippe, M. An exceptional salt-tolerant antimicrobial peptide derived from a novel gene family of haemocytes of the marine invertebrate Ciona intestinalis. Biochem. J. 2008, 416, 65–75. [Google Scholar] [CrossRef] [PubMed]
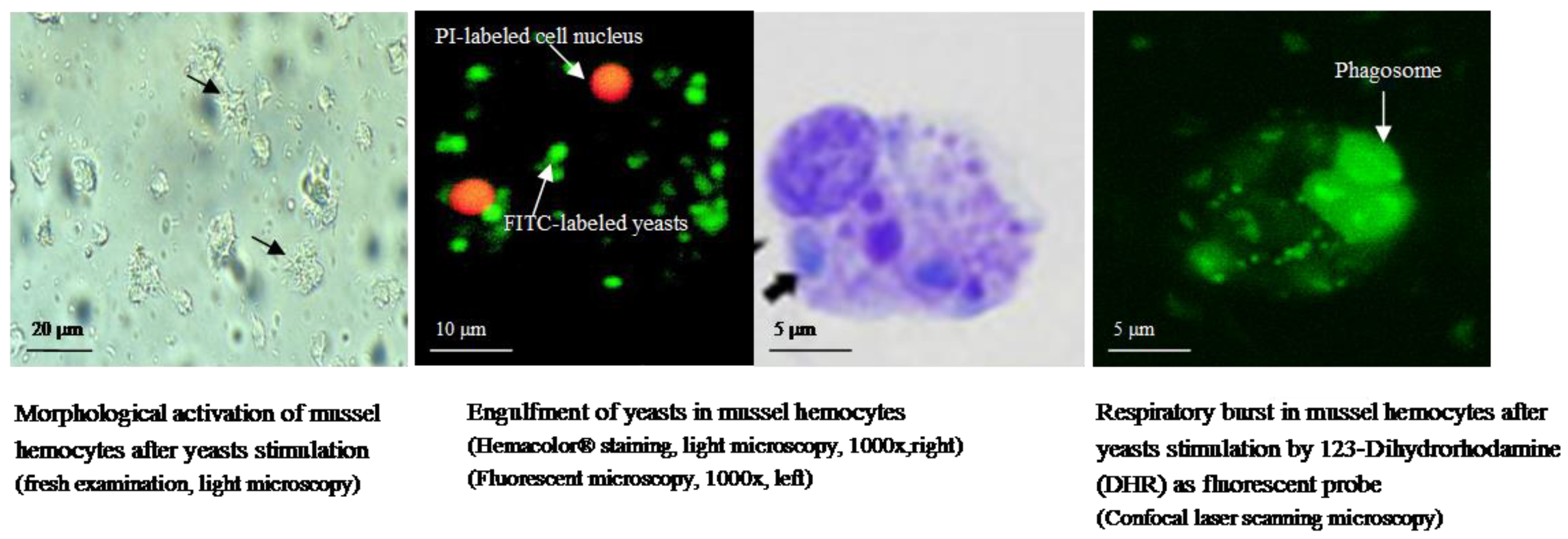
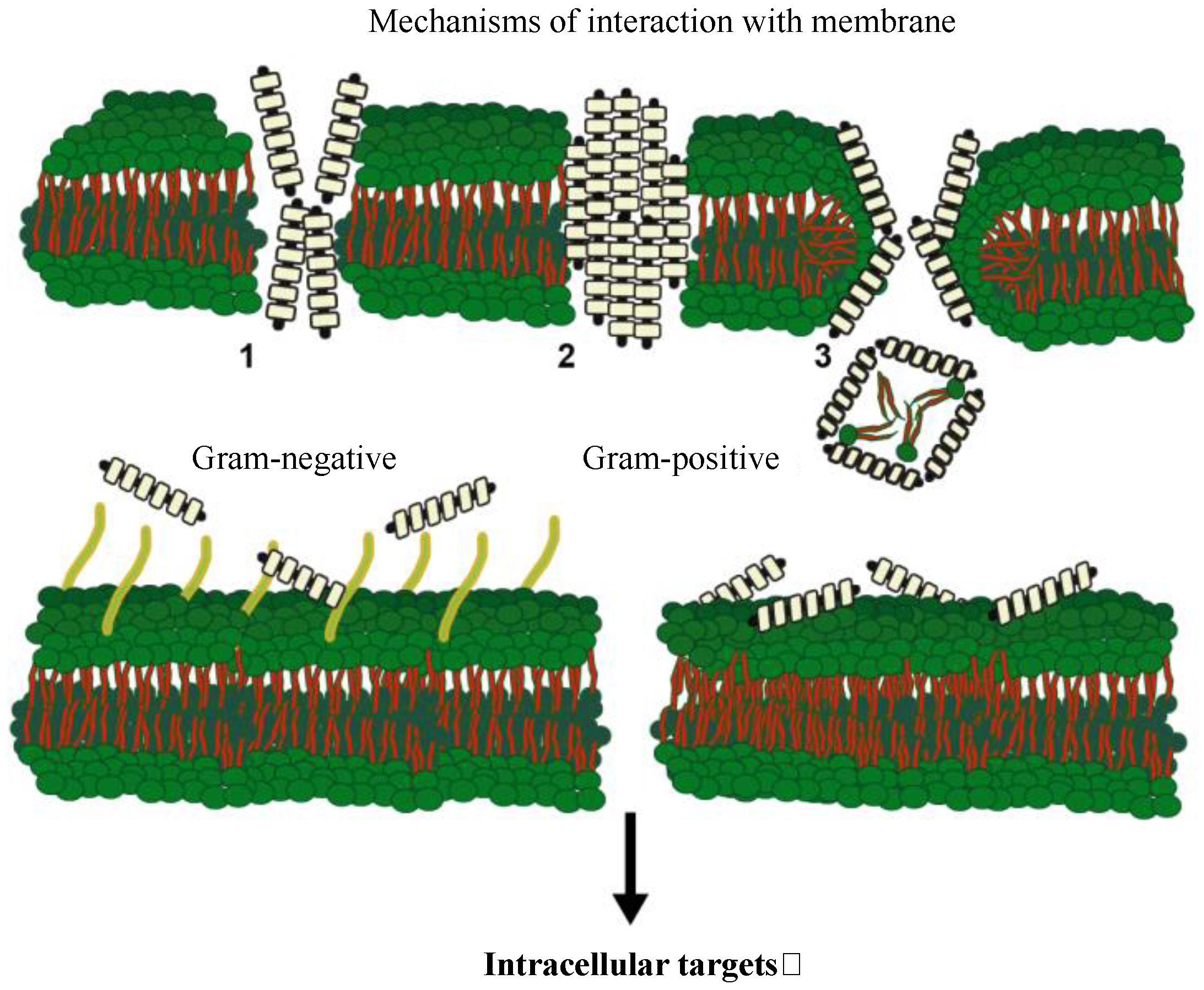
| Disease (Pathogenic Agent) | Host Species | Effects on Host | Geographical Distribution | References |
|---|---|---|---|---|
| VIRUSES | ||||
| Herpes virus infection (oyster herpes virus) | Mainly hatchery-reared larvae of Crassostrea gigas and Ostrea spp. | Velar and mantle lesions; deterioration; swim in circles | Europe (France, Ireland, Italy, The Netherlands, Spain); U.K.; Australia; New Zealand, Mexico, USA, Japan, South Korea, China | [10,13,14] |
| Gill necrosis virus (GNV) | Crassostrea angulata and C. gigas | Destruction of gill filaments | France, Portugal, Spain, U.K. | [77] |
| Hemocyte infection virus (HIV) | Virus infected hemocytes | France, Spain | ||
| Oyster velar virus disease (OVV) | C. gigas larvae | Larval movement affected through loss of infected epithelial cells from velum | Washington State, USA | |
| Disease (Pathogenic Agent) | Host Species | Effects on Host | Geographical Distribution | References |
|---|---|---|---|---|
| BACTERIA | ||||
| Larval and juvenile vibriosis (Vibrio anguillarum, V. tubiashi, V. alginolyticus, V. splendidus, V. aestuarianus, V. neptunius) | Wide range of hatchery-reared species | Tissue necrosis (due to production of exotoxin by the bacteria), up to 100% larval mortality | In all marine waters where bivalve hatchery culture is practiced | [78,79] |
| Brown ring disease (Vibrio tapetis) | Ruditapes philippinarum | Brown deposit on shell; degeneration of digestive gland followed by metabolic disorder and death | Entire European Atlantic coast to North Africa, including coasts of France, Portugal, Spain, Italy, U.K., Ireland and Norway, west coast of Korea | [30,32] |
| Roseovarius oyster disease (Roseovarius crassostreae) | Crassostrea virginica juveniles <25 mm shell length | Reduced growth rates, fragile shell development, cupping on the left valve, mantle lesions, up to 90% mortalities | USA | [37] |
| Pacific oyster nocardiosis (Nocardia crassostreae) | Crassostrea gigas, Ostrea edulis cultivated near infected C. gigas | Yellow-green pustules in the mantle, gills, adductor and cardiac muscle, up to 35% mortalities | West coast of North America from the Strait of Georgia, British Columbia to California, and Japan (Matsushima Bay), Mediterranean Sea | [39,40] |
| Disease (Pathogenic Agent) | Host Species | Effects on Host | Geographical Distribution | References |
|---|---|---|---|---|
| PROTISTS | ||||
| Bonamiasis (Bonamia ostreae, B. exitiosa, B. perspora, B. roughleyi) | Wide range of oyster species | Yellow discoloration of tissue, extensive lesions on gill and mantle, breakdown of connective tissue, significant mortality (up to 90%) | Europe, U.K., west coast Canada, east and west coasts of USA, New Zealand and SE Australia | [44,45,46,48,50,51,80] |
| Digestive gland (or Aber) disease (Marteilia refringens) | Ostrea edulis and Mytilus galloprovincialis | Pale digestive gland, severe emaciation, tissue necrosis, cessation of growth, mortalities up to 90% in summer | In O. edulis: Europe (Albania, Croatia, France, Greece, Italy, Morocco, Portugal, Spain, Sweden, Tunisia, U.K.); in M. galloprovincialis: northern Greece, in Italy, along the Adriatic Sea and the Campanian coast (Tyrrhenian Sea) | [56,57,58,59] |
| QX disease (Marteilia sydneyi) | Saccostrea glomerata and Saccostrea spp. | Necrosis of digestive gland, loss of condition, gonad absorption, mortalities up to 90% in summer | New South Wales, Queensland and Western Australia. | [60,61] |
| Dermo disease (Perkinsus marinus) | Crassostrea virginica | Severe emaciation, loss of condition, high mortality rate depending on temperature and salinity | Gulf of Mexico, southeast coast of USA, Pacific coast of Mexico, Gulf of California, Brazil | [65,66,67,68,69] |
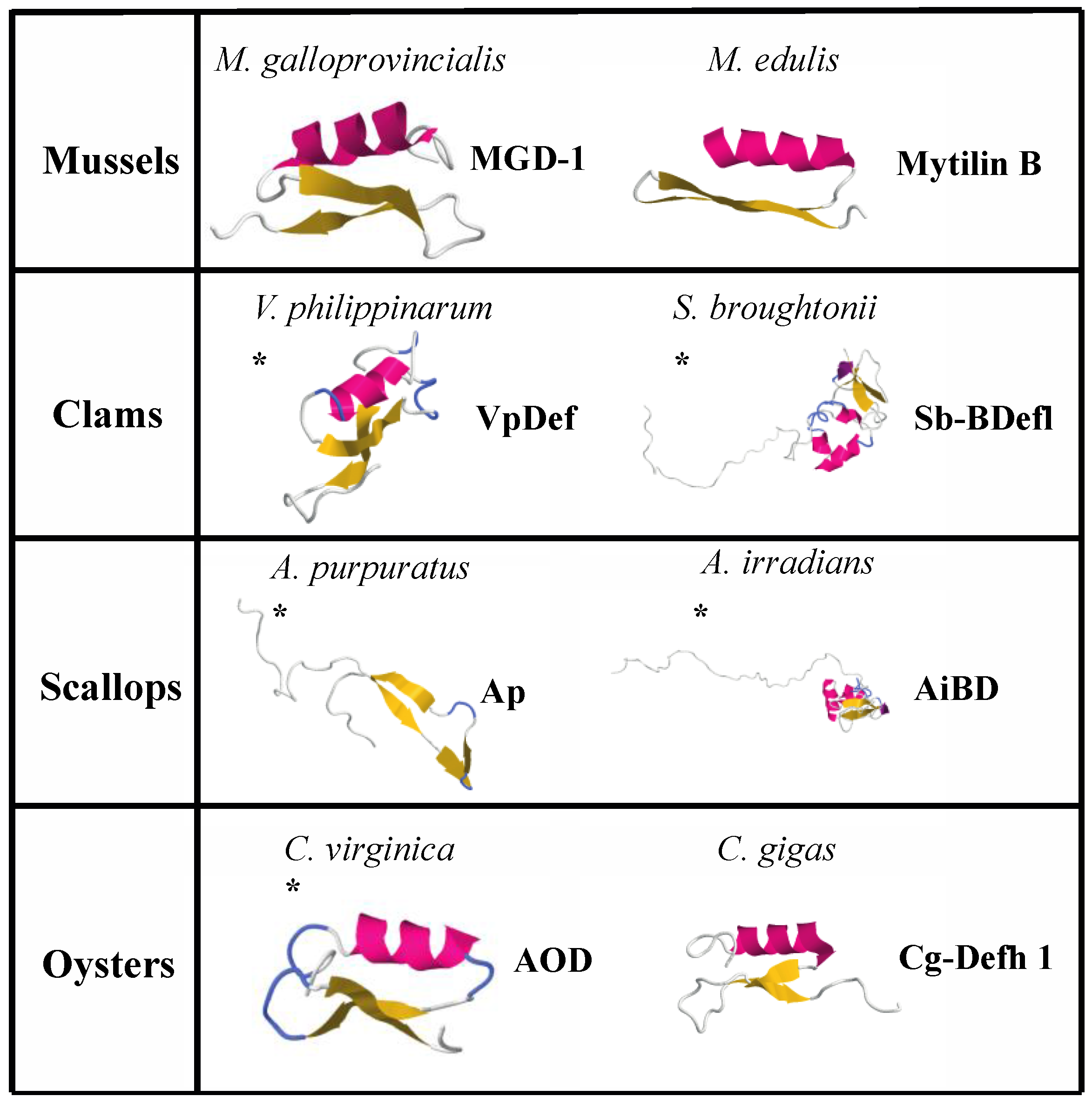
| Name | Source | Sequence | Length | Net Charge | % Hydrophobic Residues | Structure | Antimicrobial Activity | Reference |
|---|---|---|---|---|---|---|---|---|
| Defensin MGD-1 | Mytilus galloprovincialis | GFGCPNNYQCHRHCKSIPGRCGGYCGGWHRLPCTCYRCG | 39 | 5 | 30 | Combined helix and β-sheet | Gram+ | [224] |
| Defensin MGD-2 | Mytilus galloprovincialis | GFGCPNNYACHQHCKSIRGYCGGYCAGWFRLRCTCYRCG | 39 | 5 | 38 | * Combined helix and β-sheet | Gram+ and Gram− | [227] |
| Mytilin A | Mytilus edulis | GCASRCKAKCAGRRCKGWASASFRGRCYCKCFRC | 34 | 10 | 47 | * Combined helix and β-sheet | Gram+ and Gram− | [225] |
| Mytilin B | Mytilus edulis | SCASRCKGHCRARRCGYYVSVLYRGRCYCKCLRC | 34 | 9 | 41 | Combined helix and β-sheet | Gram+ and Gram−, antiviral | [225] |
| Myticin A | Mytilus galloprovincialis | HSHACTSYWCGKFCGTASCTHYLCRVLHPGKMCACVHCSR | 40 | 4 | 45 | * Combined helix and β-sheet | Gram+ and Gram−; antifungal | [226] |
| Myticin B | Mytilus galloprovincialis | HPHVCTSYYCSKFCGTAGCTRYGCRNLHRGKLCFCLHCSR | 40 | 6 | 37 | * Combined helix and β-sheet | Gram+ and Gram−; antifungal | [226] |
| Myticin C | Mytilus galloprovincialis | QSVACTSYYCSKFCGSAGCSLYGCYLLHPGKICYCLHCSR | 40 | 3 | 35 | * Combined helix and β-sheet | Gram+ and Gram−; antifungal | [239] |
| Mytimycin | Mytilus galloprovincialis | MSLVLRMTLLFVVCCVVIGMSNAACCHKPFWKHCWDCTAGTPYCGYRSCNIFGCGCTCRTEPYGKSCYERGNRCRCYTDKRKRRSLSFEDISPNIKFAGLDINSDGLIEQFEFIKALEQMDIIDNTTMFHHWSIMDEDKDGTITLEEFDK | 150 | −2 | 41 | * Combined helix and β-sheet | Antifungal | [225] |
| Mytimacin | Mytilus galloprovincialis | MGYIGLCGVLLSLSLLMLLQIPTSDANVLGDCWEDWSRCTRQTNWFTNIAWQSCPNRCKCQGHAGGNCIQVRSNCFLWRNKRWMCNCYGRRSGPKPGWCGF | 101 | 7 | 43 | * Combined helix and β-sheet | Gram+ and Gram− | [243] |
| Big-Defensin | Mytilus galloprovincialis | MNRKAILCVLYATLLIIPAPILGRVVAKKKEEKRYAAVYP IAAYAGMTVSLPVFLALVAAYGAWTVARYHIRSRSRSSSHNSHNCANNRGWCRPNCFRREYHDWYHSDTCGSYKCCRYR | 119 | 14 | 42 | * Combined helix and β-sheet | Gram+ and Gram− | [243] |
| Myticusin-1 | Mytilus coruscus | TDHQMAQSACIGVSQDNAYASAIPRDCHGGKTCEGICADATATMDRYSDTGGPLSIARCVNAFHFYKRRGEENVSYKPFVVSWKYGVAGCFYTHCGPNFCCCIS | 104 | 0 | 39 | * Combined helix and β-sheet | Gram+ and Gram−, antifungal | [241] |
| VpBD | Venerupis philippinarum | LCLDQKPEMEPFRKDAQQALEPSRQRRWLHRRCLSGRGFCRAICSIFEEPVRGNIDCYFGYNCCRRMFSHYRTS | 74 | 5 | 36 | * Helix | Gram+ and Gram− | [244] |
| MCdef | Ruditapes philippinarum | GFGCPNDYSCSNHCRDSIGCRGGYCKYQLICTCYGCKKRRSIQE | 44 | 4 | 29 | * Combined helix and β-sheet | Gram+ and Gram− | [133] |
| VpDef | Venerupis philippinarum | GFGCPEDEYECHNHCKNSVGCRGGYCDAGTLRQRCTCYGCNQKGRSIQE | 49 | 0 | 26 | * Combined helix and β-sheet | Gram+ and Gram− | [162] |
| Sb-BDef1 | Scapharca broughtonii | MTHKIVLCCIYLLLSTSFILSKHLPEERKQKKQVLLAAGA GVALSELLGPVLVGAGTLAGAALLNQAVSSNRWVIPCANNRGWCRTDCHFGEHIDDYHSD ICHSGYKCCRY | 111 | 3 | 45 | * Combined helix and β-sheet | Gram− | [245] |
| Ap | Argopecten purpuratus | TYMPVEEGEYIVNISYADQPKKNSPFTAKKQPGPKVDLSGVKAYGPG | 47 | 1 | 25 | * Polyproline rich β-sheet | Gram+, antifungal | [254] |
| AiBD | Argopecten irradians | MTRPSLVRCYSLFFTALIVMAIICPAWSEEIPKSRKKRAIPIAYVGMAVAPQVFRWLVRAYGAAAVTAAGVTLRRVINRSRSNDNHSCYGNRGWCRSSCRSYEREYRGGNLGVCGSYKCCVT | 122 | 14 | 44 | * Combined helix and β-sheet | Gram+ and Gram−, antifungal | [253] |
| AOD | Crassostrea virginica | GFGCPWNRYQCHSHCRSIGRLGGYCAGSLRLTCTCYRS | 38 | 5 | 34 | * Combined helix and β-sheet | Gram+ and Gram− | [247] |
| Cg-Prp | Crassostrea gigas | ILENLLARSTNEDREGSIFDTGPIRRPKPRPRPRPEG | 37 | 2 | 21 | * Proline-rich peptide | Synergistic antimicrobial activity with Cg-Def | [248] |
| cgMolluscidin | Crassostrea gigas | AATAKKGAKKADAPAKPKKATKPKSPKKAAKKAGAKKGVKRAGKKGAKKTTKAKK | 55 | 23 | 29 | * Helix | Gram+ and Gram− | [247] |
| Cg-Defh1 | Crassostrea gigas | GFGCPRDQYKCNSHCQSIGCRAGYCDAVTLWLRCTCTDCNGKK | 43 | 3 | 37 | Combined helix and β-sheet | Gram+ and Gram− | [249] |
| Cg-Defh2 | Crassostrea gigas | GFGCPGDQYECNRHCRSIGCRAGYCDAVTLWLRCTCTGCSGKK | 43 | 3 | 37 | * Combined helix and β-sheet | Gram+ and Gram− | [229] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Mar. Drugs 2017, 15, 182. https://doi.org/10.3390/md15060182
Zannella C, Mosca F, Mariani F, Franci G, Folliero V, Galdiero M, Tiscar PG, Galdiero M. Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Marine Drugs. 2017; 15(6):182. https://doi.org/10.3390/md15060182
Chicago/Turabian StyleZannella, Carla, Francesco Mosca, Francesca Mariani, Gianluigi Franci, Veronica Folliero, Marilena Galdiero, Pietro Giorgio Tiscar, and Massimiliano Galdiero. 2017. "Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense" Marine Drugs 15, no. 6: 182. https://doi.org/10.3390/md15060182
APA StyleZannella, C., Mosca, F., Mariani, F., Franci, G., Folliero, V., Galdiero, M., Tiscar, P. G., & Galdiero, M. (2017). Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Marine Drugs, 15(6), 182. https://doi.org/10.3390/md15060182






