Isoprenoids from the Soft Coral Sarcophyton glaucum
Abstract
:1. Introduction
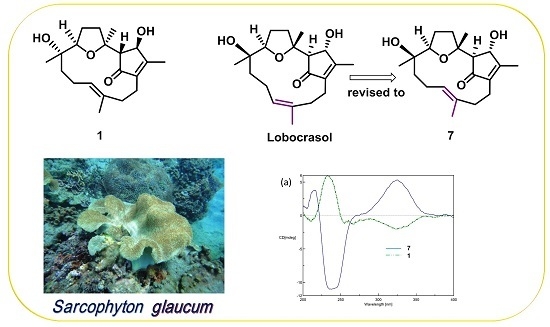
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, W.Y.; Chen, B.W.; Huang, C.Y.; Wen, Z.H.; Sung, P.J.; Su, J.H.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids, sarcocrassocolides P−R, from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Lu, Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Bioactive cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Wang, S.K.; Chung, S.G.; Chou, G.C.; Dai, C.F. Cytotoxic cembrenolides and steroids from the formosan soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.L.; Li, G.Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Su, J.; Liang, Y.; Yang, X.; Zheng, K.; Zeng, L. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- ElAhwany, A.M.D.; Ghozlan, H.A.; ElSharif, H.A.; Sabry, S.A. Phylogenetic diversity and antimicrobial activity of marine bacteria associated with the soft coral Sarcophyton glaucum. J. Basic Microbiol. 2015, 55, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Fleury, B.G.; Coll, J.C.; Sammarco, P.W. Complementary (secondary) metabolites in a soft coral: Sex-specific variability, inter-clonal variability, and competition. Mar. Ecol. 2006, 27, 204–218. [Google Scholar] [CrossRef]
- Néeman, I.; Fishelson, L.; Kashman, Y. Sarcophine—A new toxin from the soft coral Sarcophyton glaucum (Alcyonaria). Toxicon 1974, 12, 593–598. [Google Scholar] [CrossRef]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Wang, S.K.; Cheng, S.Y.; Duh, C.Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009, 11, 3012–3014. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.A.E.; Hamann, M.T. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. J. Org. Chem. 1998, 63, 7449–7455. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Wang, S.K.; Duh, C.Y. Cembranoids from the Dongsha Atoll soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Tsai, C.R.; Huang, C.Y.; Wang, S.Y.; Sheu, J.H. Klyflaccicembranols A–I, new cembranoids from the soft coral Klyxum flaccidum. Mar. Drugs 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Gong, H.L.; Yao, L.G.; Guo, Y.W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.B.; Xiao, H.; Fan, C.Q.; Lu, Y.N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; He, X.X.; Zhang, J.; Guo, Q.; Lei, L.F.; Su, J.Y.; Zeng, L.M. New precursor of tetraterpenoids from the soft coral Sarcophyton glaucum. Nat. Prod. Res. 2013, 27, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.; Porte, A.L. The structure of loliolide: A terpene from lolium perenne. Tetrahedron 1964, 20, 1463–1467. [Google Scholar] [CrossRef]
- Lin, S.; Shi, T.; Chen, K.Y.; Zhang, Z.X.; Shan, L.; Shen, Y.H.; Zhang, W.D. Cyclopenicillone, a unique cyclopentenone from the cultures of Penicillium decumbens. Chem. Comm. 2011, 47, 10413–10415. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.B.; Shin, J. Meroditerpenoids from the Brown Alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Carroll, A.R.; Quinn, R.J. Longithorols C–E, Three new macrocyclic sesquiterpene hydroquinone metabolites from the Australian ascidian, Aplidium longithorax. J. Nat. Prod. 1999, 62, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Youssef, D.T.A.; Reiland, J.; Ferniz, M.; Marchetti, D.; El Sayed, K.A. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J. Nat. Prod. 2006, 69, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J. Sarcophine, a new epoxy cembranolide from Marine origin. Tetrahedron 1974, 30, 2817–2824. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Sung, C.S.; Lan, Y.H.; Wang, Y.C.; Lu, M.C.; Wen, Z.H.; Wu, Y.C.; Sung, P.J. New anti-inflammatory cembranes from the cultured soft coral Nephthea columnaris. Mar. Drugs 2015, 13, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.G.; Liu, H.L.; Guo, Y.W.; Mollo, E. New cembranoids from the Hainan soft coral Sarcophyton glaucum. Helv. Chim. Acta 2009, 92, 1085–1091. [Google Scholar] [CrossRef]





| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 142.8 (C) | 71.9 (C) | 152.2 (C) | 165.3 (C) |
| 2 | 204.3 (C) | 76.6 (CH) | 148.2 (C) | 79.6 (CH) |
| 3 | 59.4 (CH) | 122.4 (CH) | 116.5 (CH) | 119.5 (CH) |
| 4 | 83.2 (C) | 140.0 (C) | 74.2 (C) | 143.8 (C) |
| 5 | 32.4 (CH2) | 37.9 (CH2) | 38.8 (CH2) | 35.7 (CH2) |
| 6 | 25.0 (CH2) | 25.4 (CH2) | 26.9 (CH2) | 24.2 (CH2) |
| 7 | 83.8 (CH) | 61.9 (CH) | 74.6 (CH) | 83.4 (CH) |
| 8 | 73.6 (C) | 59.8 (C) | 74.9 (C) | 68.3 (C) |
| 9 | 34.0 (CH2) | 40.2 (CH2) | 38.0 (CH2) | 40.3 (CH2) |
| 10 | 21.5 (CH2) | 23.7 (CH2) | 22.1 (CH2) | 23.0 (CH2) |
| 11 | 128.4 (CH) | 123.9 (CH) | 128.5 (CH) | 80.0 (CH) |
| 12 | 133.3 (C) | 136.1 (C) | 131.4 (C) | 71.3 (C) |
| 13 | 39.1 (CH2) | 35.2 (CH2) | 38.3 (CH2) | 36.8 (CH2) |
| 14 | 20.0 (CH2) | 27.5 (CH2) | 22.3 (CH2) | 20.1 (CH2) |
| 15 | 167.0 (C) | 67.4 (C) | 123.7 (C) | 121.2 (C) |
| 16 | 75.9 (CH) | 69.4 (CH2) | 170.3 (C) | 174.5 (C) |
| 17 | 13.2 (CH3) | 12.1 (CH3) | 8.8 (CH3) | 8.5 (CH3) |
| 18 | 25.2 (CH3) | 15.6 (CH3) | 29.5 (CH3) | 16.2 (CH3) |
| 19 | 25.8 (CH3) | 16.7 (CH3) | 25.5 (CH3) | 20.0 (CH3) |
| 20 | 15.3 (CH3) | 14.9 (CH3) | 15.9 (CH3) | 23.4 (CH3) |
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | - | 4.87 d (11.2) | - | 5.53 d (10.4) |
| 3 | 2.16 br s | 5.26 dd (10.8, 0.8) | 5.21 s | 4.99 d (10.4) |
| 5 | 3.04 q (10.8) | 2.34 m | 2.09 m | 2.18 m |
| 1.67 m | - | 1.78 m | - | |
| 6 | 2.00 dd (9.6, 2.4) | 1.92 m | 1.54 m | 1.97 m |
| 1.54 m | 1.63 m | 1.51 m | 1.42 m | |
| 7 | 3.80 dd (9.2, 5.6) | 2.65 t (4.0) | 3.52 dd (9.2, 3.2) | 3.01 dd (10.0, 2.8) |
| 9 | 1.50 m | 2.13 m | 1.64 m | 1.73 m |
| 1.25 m | 0.93 m | 1.47 m | 1.49 m | |
| 10 | 2.05 m | 2.27 m | 2.17 m | 1.62 m |
| 1.90 m | 1.87 m | 1.86 m | 1.34 m | |
| 11 | 5.15 t (7.6) | 5.11 dd (9.6, 5.6) | 4.99 t (6.8) | 3.13 d (10.4) |
| 13 | 2.28 br d (13.2) | 2.22 m | 2.28 m | 1.70 m |
| 1.84 m | 1.95 m | - | 1.49 m | |
| 14 | 2.61 td (13.2, 2.1) | 1.69 m | 2.56 m | 2.45 m |
| 2.12 m | - | - | 1.91 m | |
| 16 | 4.90 br s | 3.89 d (10.4) | - | - |
| - | 3.75 d (10.4) | - | - | |
| 17 | 2.08 s | 1.43 s | 1.94 s | 1.73 s |
| 18 | 1.35 s | 1.84 s | 1.40 s | 1.77 s |
| 19 | 1.03 s | 1.27 s | 1.61 s | 0.98 s |
| 20 | 1.69 s | 1.57 s | 1.67 s | 0.98 s |
| No. | δH (J in Hz) a | δC (mult.) b | No. | δH (J in Hz) a | δC (mult.) b |
|---|---|---|---|---|---|
| 1 | 1.99 m | 35.2 (CH2) | 15 | 1.62 m | 36.5 (CH2) |
| 1.54 m | - | - | 1.45 m | - | |
| 2 | 1.75 m | 32.0 (CH2) | 16 | 4.62 d (5.2) | 73.0 (CH) |
| 1.51 m | - | 17 | - | 148.2 (C) | |
| 3 | 4.00 m | 68.2 (CH) | 18 | 0.91 s | 18.8 (CH3) |
| 4 | 2.09 dd (13.2, 11.6) | 42.0 (CH2) | 19 | 1.28 s | 17.5 (CH3) |
| 1.54 m | - | 20 | - | 134.2 (C) | |
| 5 | - | 77.4 (C) | 21 | 1.77 s | 17.5 (CH3) |
| 6 | 3.47 br s | 76.6 (CH) | 22 | 2.41 dd (13.2, 10.8) | 43.0 (CH2) |
| 7 | 1.84 m | 35.3 (CH2) | - | 1.91 m | - |
| 8 | 1.88 m | 29.1 (CH) | 23 | 1.86 m | 34.0 (CH) |
| 9 | 1.57 m | 53.3 (CH) | 24 | 1.09 m | 45.9 (CH) |
| 10 | - | 41.1 (C) | 25 | 1.64 m | 31.3 (CH) |
| 11 | 3.88 td (10.4, 5.2) | 69.6 (CH) | 26 | 0.87 d (6.8) | 19.6 (CH3) |
| 12 | 2.62 dd (12.0, 5.2) | 50.2 (CH2) | 27 | 0.93 d (6.8) | 22.0 (CH3) |
| 1.58 m | - | 28 | 0.82 d (6.8) | 11.9 (CH3) | |
| 13 | - | 46.0 (C) | 29 | 0.72 d (6.8) | 13.9 (CH3) |
| 14 | 1.78 m | 52.5 (CH) | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-H.; Li, W.-L.; Huang, C.-Y.; Ahmed, A.F.; Dai, C.-F.; Wu, Y.-C.; Lu, M.-C.; Liaw, C.-C.; Sheu, J.-H. Isoprenoids from the Soft Coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. https://doi.org/10.3390/md15070202
Chao C-H, Li W-L, Huang C-Y, Ahmed AF, Dai C-F, Wu Y-C, Lu M-C, Liaw C-C, Sheu J-H. Isoprenoids from the Soft Coral Sarcophyton glaucum. Marine Drugs. 2017; 15(7):202. https://doi.org/10.3390/md15070202
Chicago/Turabian StyleChao, Chih-Hua, Wen-Liang Li, Chiung-Yao Huang, Atallah F. Ahmed, Chang-Feng Dai, Yang-Chang Wu, Mei-Chin Lu, Chih-Chuang Liaw, and Jyh-Horng Sheu. 2017. "Isoprenoids from the Soft Coral Sarcophyton glaucum" Marine Drugs 15, no. 7: 202. https://doi.org/10.3390/md15070202
APA StyleChao, C.-H., Li, W.-L., Huang, C.-Y., Ahmed, A. F., Dai, C.-F., Wu, Y.-C., Lu, M.-C., Liaw, C.-C., & Sheu, J.-H. (2017). Isoprenoids from the Soft Coral Sarcophyton glaucum. Marine Drugs, 15(7), 202. https://doi.org/10.3390/md15070202