Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark (Galeus melastomus)
Abstract
:1. Introduction
2. Results and Discussion
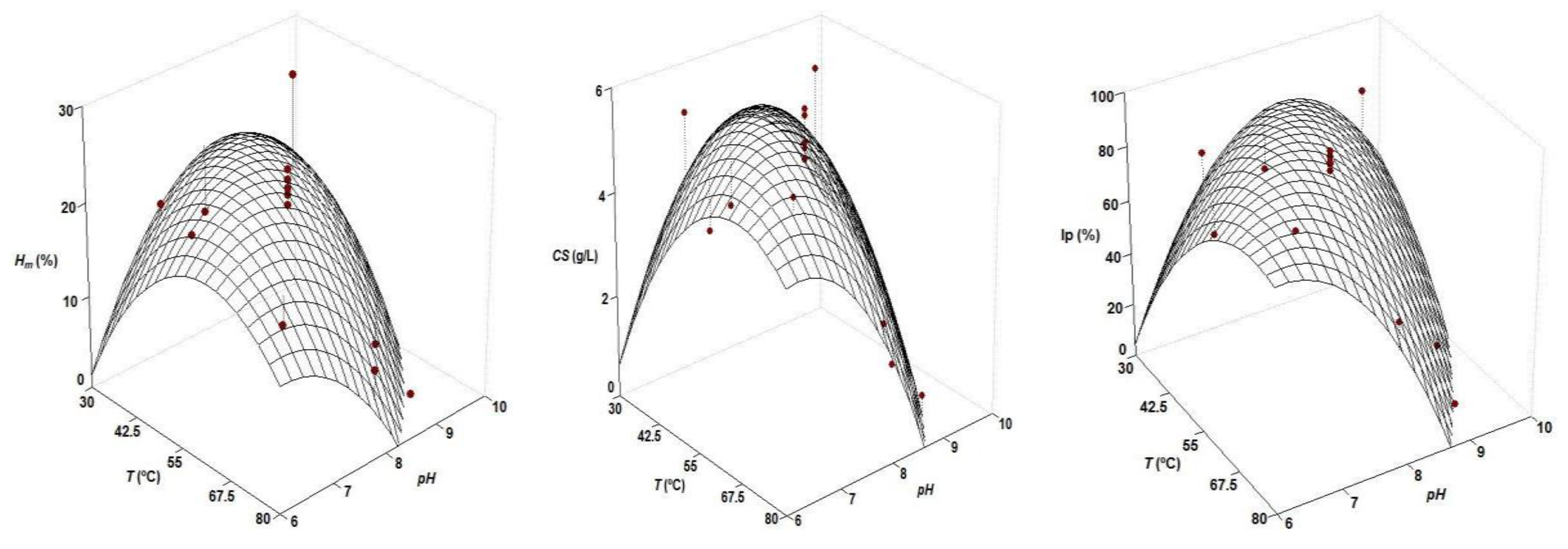
2.1. Hydrolysis of Cartilage by Enzyme Proteolysis
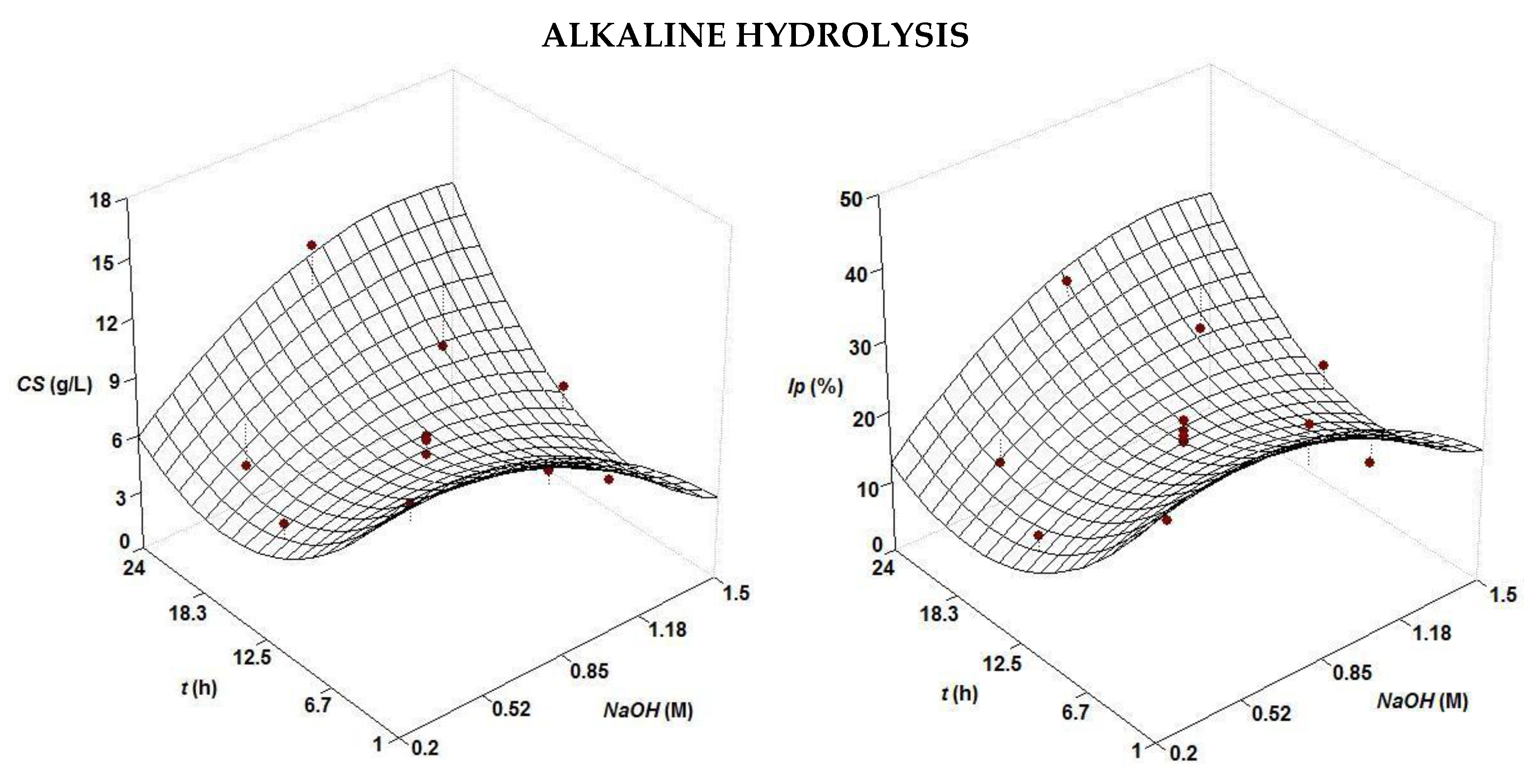
2.2. Isolation of CS by Chemical Treatments
2.3. Diafiltration for CS Purification
2.4. Molecular Weight of CS
2.5. Composition of CS
3. Experimental Section
3.1. Preparation of Cartilage and Proximal and Analytical Determinations
3.2. Factorial Designs and Statistical Analysis
3.3. Cartilage Enzymatic Digestion
3.4. Chemical Processing of the Hydrolysates
3.5. Purification of CS by UF-DF
3.6. Molecular Weight of CS
3.7. CS Composition by 1H NMR and SAX-HPLC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Rudd, T.; Yates, E. New applications of heparin and other glycosaminoglycans. Molecules 2017, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. A dilemma in the glycosaminoglycan-based therapy: Synthetic or naturally unique molecules? Med. Res. Rev. 2015, 35, 1195–1219. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Özbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Basappa; Murugan, S.; Sugahara, K.N.; Lee, C.M.; ten Dam, G.B.; van Kuppevelt, T.H.; Miyasaka, M.; Yamada, S.; Sugahara, K. Involvement of chondroitin sulfate E in the liver tumor focal formation of murine osteosarcoma cells. Glycobiology 2009, 19, 735–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Wei, Y.-H.; Chu, I.M.; Yao, C.-L. Effect of chondroitin sulphate C on the in vitro and in vivo chondrogenesis of mesenchymal stem cells in crosslinked type II collagen scaffolds. J. Tissue Eng. Regen. Med. 2013, 7, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nakajima, T.; Yang, J.J.; Kurokawa, T.; Liu, J.; Lu, J.; Mizumoto, S.; Sugahara, K.; Kitamura, N.; Yasuda, K.; et al. Proteoglycans and glycosaminoglycans improve toughness of biocompatible double network hydrogels. Adv. Mater. 2014, 26, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Nandini, C.D.; Mikami, T.; Ohta, M.; Itoh, N.; Akiyama-Nambu, F.; Sugahara, K. Structural and functional characterization of oversulfated chondroitin sulfate/dermatan sulfate hybrid chains from the notochord of hagfish: Neuritogenic and binding activities for growth factors and neurotrophic factors. J. Biol. Chem. 2004, 279, 50799–50809. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, E.O.; Gomes, A.M.; Silva, C.S.; Pereira, M.S.; de Vilela Silva, A.C.E.S.; Pavão, M.S.G. Structure and biological activities of glycosaminoglycan analogs from marine invertebrates: New therapeutic agents? In Glycans in Diseases and Therapeutics; Pavão, S.G.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 159–184. [Google Scholar]
- Antelo, L.T.; de Hijas-Liste, G.M.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R.I. Optimisation of processing routes for a marine biorefinery. J. Clean. Prod. 2015, 104, 489–501. [Google Scholar] [CrossRef] [Green Version]
- EC (European Commission). Regulation (EU) No 1380/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Fisheries Policy, Amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and Repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC, 2013. Available online: http://data.europa.eu/eli/reg/2013/1380/oj (accessed on 5 September 2018).
- Ordóñez-Del Pazo, T.; Antelo, L.T.; Franco-Uría, A.; Pérez-Martín, R.I.; Sotelo, C.G.; Alonso, A.A. Fish discards management in selected Spanish and Portuguese métiers: Identification and potential valorisation. Trends Food Sci. Technol. 2014, 36, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Berrow, S. Incidental capture of elasmobranchs in the bottom-set gill-net fishery off the south coast of Ireland. J. Mar. Biol. Assoc. UK 1994, 74, 837–848. [Google Scholar]
- Krylov, V.B.; Grachev, A.A.; Ustyuzhanina, N.E.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Kozlova, N.I.; Portsel, M.N.; Konovalova, I.N.; Novikov, V.Y.; Siebert, H.-C.; et al. Preliminary structural characterization, anti-inflammatory and anticoagulant activities of chondroitin sulfates from marine fish cartilage. Russ. Chem. Bull. 2011, 60, 746. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.; Fraguas, J.; González, M.; Murado, M.Á. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Kim, J.-G.; Je, J.-Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of fish protein hydrolysates from Scyliorhinus canicula discards with antihypertensive and antioxidant activities by enzymatic hydrolysis and mathematical optimization using response surface methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Pérez-Martín, R.I. Optimization of high purity chitin and chitosan production from Illex argentinus pens by a combination of enzymatic and chemical processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Ji, C.I.; Woo, J.W.; Do, J.R.; Cho, S.M.; Lee, Y.B.; Kang, S.N.; Park, J.H. Simplified purification of chondroitin sulphate from scapular cartilage of shortfin mako shark (Isurus oxyrinchus). Int. J. Food Sci. Technol. 2012, 47, 91–99. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.; Vázquez, J.A.; Ferreira, I.C. Optimization of microwave-assisted extraction of hydrophilic and lipophilic antioxidants from a surplus tomato crop by response surface methodology. Food Bioprod. Process. 2016, 98, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y. Optimization of extraction conditions and characterization of pepsin-solubilised collagen from skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, S.H.; Ramani, K. Microbial assisted industrially important multiple enzymes from fish processing waste: Purification, characterization and application for the simultaneous hydrolysis of lipid and protein molecules. RSC Adv. 2016, 6, 93602–93620. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the preparation of fish protein anti-obesity hydrolysates using response surface methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Murado, M.A.; Fraguas, J.; Montemayor, M.I.; Vázquez, J.A.; González, P. Preparation of highly purified chondroitin sulphate from skate (Raja clavata) cartilage by-products. Process optimization including a new procedure of alkaline hydroalcoholic hydrolysis. Biochem. Eng. J. 2010, 49, 126–132. [Google Scholar] [CrossRef]
- Shi, Y.G.; Meng, Y.C.; Li, J.R.; Chen, J.; Liu, Y.H.; Bai, X. Chondroitin sulfate: Extraction, purification, microbial and chemical synthesis. J. Chem. Technol. Biotechnol. 2014, 89, 1445–1465. [Google Scholar] [CrossRef]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Lignot, B.; Lahogue, V.; Bourseau, P. Enzymatic extraction of chondroitin sulfate from skate cartilage and concentration-desalting by ultrafiltration. J. Biotechnol. 2003, 103, 281–284. [Google Scholar] [CrossRef]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.; Vázquez, J.A.; González, M. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Lamarque, G.; Lucas, J.-M.; Viton, C.; Domard, A. Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: Role of various structural parameters. Biomacromolecules 2005, 6, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ottøy, M.H.; Vårum, K.M.; Christensen, B.E.; Anthonsen, M.W.; Smidsrød, O. Preparative and analytical size-exclusion chromatography of chitosans. Carbohydr. Polym. 1996, 31, 253–261. [Google Scholar] [CrossRef]
- Bertini, S.; Bisio, A.; Torri, G.; Bensi, D.; Terbojevich, M. Molecular weight determination of heparin and dermatan sulfate by size exclusion chromatography with a triple detector array. Biomacromolecules 2005, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of low molecular weight chondroitin sulfates, screening of a high anti-complement capacity of low molecular weight chondroitin sulfate and its biological activity studies in attenuating osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: A valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. NMR chemical shifts in structural biology of glycosaminoglycans. Anal. Chem. 2013, 86, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Volpi, N. Selective removal of keratan sulfate in chondroitin sulfate samples by sequential precipitation with ethanol. Anal. Biochem. 2014, 448, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Purification of heparin, dermatan sulfate and chondroitin sulfate from mixtures by sequential precipitation with various organic solvents. J. Chromatogr. B Biomed. Sci. Appl. 1996, 685, 27–34. [Google Scholar] [CrossRef]
- Mucci, A.; Schenetti, L.; Volpi, N. 1H and 13C nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr. Polym. 2000, 41, 37–45. [Google Scholar] [CrossRef]
- Pomin, V.H.; Park, Y.; Huang, R.; Heiss, C.; Sharp, J.S.; Azadi, P.; Prestegard, J.H. Exploiting enzyme specificities in digestions of chondroitin sulfates A and C: Production of well-defined hexasaccharides. Glycobiology 2012, 22, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, K.; Takeuchi, Y.; Mukuno, A.; Tomitori, H.; Miya, M.; Linhardt, R.J.; Toida, T. Composition of glycosaminoglycans in elasmobranchs including several deep-sea sharks: Identification of chondroitin/dermatan sulfate from the dried fins of Isurus oxyrinchus and Prionace glauca. PLOS ONE 2015, 10, e0120860. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-S.; Huang, J.-P.; Huang, C.-W.; Chu, I.M. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J. Biosci. Bioeng. 2009, 107, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Liu, H.-C.; Lin, C.-C.; Chou, C.-H.; Lin, F.-H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hu, Y.; Hao, W.; Han, Y.; Meng, G.; Zhang, D.; Wu, Z.; Wang, H. A novel injectable scaffold for cartilage tissue engineering using adipose—Derived adult stem cells. J. Orthop. Res. 2008, 26, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite hydrogel of chitosan–poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Colloids Surf. B. Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.H.; Chan, S.-W.; Li, J.; Goh, J.C.H.; Li, L.; Ren, X.F.; Lee, E.H. Intra-articular delivery of chondroitin sulfate for the treatment of joint defects in rabbit model. J. Mol. Histol. 2007, 38, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.-K.; Tabata, Y. Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation. Acta Biomater. 2014, 10, 2684–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; AOAC: Washington DC, USA, 1997. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- van den Hoogen, B.M.; van Weeren, P.R.; Lopes-Cardozo, M.; van Golde, L.M.; Barneveld, A.; van de Lest, C.H. A microtiter plate assay for the determination of uronic acids. Anal. Biochem. 1998, 257, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Murado, M.A.; Vázquez, J.A.; Montemayor, M.I.; Cabo, M.L.; de Pilar González, M. Two mathematical models for the correction of carbohydrate and protein interference in the determination of uronic acids by the m-hydroxydiphenyl method. Biotechnol. Appl. Biochem. 2005, 41, 209–216. [Google Scholar] [PubMed]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery; Wiley-Interscience: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: London, UK, 1986. [Google Scholar]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidant activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Volpi, N. Hyaluronic acid and chondroitin sulfate unsaturated disaccharides analysis by high-performance liquid chromatography and fluorimetric detection with dansylhydrazine. Anal. Biochem. 2000, 277, 19–24. [Google Scholar] [CrossRef] [PubMed]




| Coded Values | Natural Values | |||||
|---|---|---|---|---|---|---|
| Enzyme Hydrolysis | NaOH Treatment | NaOH-EtOH Treatment | ||||
| pH | T (°C) | NaOH (M) | Time (h): t | NaOH (M) | Ethanol (v) | |
| −1.41 | 6.0 | 30.0 | 0.20 | 1.0 | 0.10 | 0.30 |
| −1 | 6.6 | 37.3 | 0.39 | 4.4 | 0.20 | 0.46 |
| 0 | 8.0 | 55.0 | 0.85 | 12.5 | 0.45 | 0.85 |
| +1 | 9.4 | 72.7 | 1.31 | 20.6 | 0.70 | 1.24 |
| +1.41 | 10.0 | 80.0 | 1.50 | 24.0 | 0.80 | 1.40 |
| Treatment | Second Order Equations | NaOHopt (M) | topt (h) | |
| Alkaline | CS (g/L) = 6.42 + 1.34 t NaOH − 0.88 NaOH2 + 1.68 t2 | 0.687 | 0.85 | 1 or 24 |
| Ip (%) = 19.05 + 3.03 t NaOH − 2.61 NaOH2 + 4.37 t2 | 0.709 | 0.85 | 1 or 24 | |
| NaOHopt (M) | EtOHopt (v) | |||
| Alkaline-alcoholic | CS (g/L) = 6.56 + 1.91 EtOH − 2.39 NaOH2 − 1.28 EtOH2 | 0.742 | 0.45 | 1.14 |
| Ip (%) = 67.0 + 20.90 EtOH − 20.06 NaOH2 − 13.03 EtOH2 | 0.710 | 0.45 | 1.16 |
| Second Order Equations | Topt (°C) | pHopt | |
|---|---|---|---|
| Hm (%) = 22.02 − 5.18 T − 4.82 pH − 5.56 T pH − 4.26 T2 − 4.44 pH2 | 0.801 | 47.5 | 7.61 |
| CS (g/L) = 5.25 − 0.80 T − 1.36 pH − 1.20 T pH − 0.80 T2 − 1.16 pH2 | 0.796 | 53.3 | 7.25 |
| Ip (%) = 85.06 − 11.81 T − 23.06 pH − 22.76 T pH − 10.59 T2 − 20.02 pH2 | 0.890 | 57.8 | 7.06 |
| Alkaline Hydrolysis 1 h | Alkaline Hydrolysis 24 h | Hydroalcoholic Alkaline Precipitation | |
|---|---|---|---|
| Mn | 66 kDa | 53 kDa | 55 kDa |
| PDI | 1.14 | 1.25 | 1.26 |
| CS-A (GlcA-GalNAc 4S)1 | 23.9 | 22.78 | 23.01 |
| CS-A (GlcA-GalNAc 4S)2 | 23.43 ± 0.23 | 23.52 ± 0.11 | 23.77 ± 0.13 |
| CS-C (GlcA-GalNAc 6S)2 | 54.78 ± 0.02 | 55.11 ± 0.16 | 54.93 ± 0.36 |
| CS-0 (GlcA-GalNAc 0S)2 | 3.96 ± 0.03 | 3.92 ± 0.27 | 4.23 ± 0.55 |
| CS-D (GlcA 2S-GalNAc 6S)2 | 15.75 ± 0.19 | 15.37 ± 0.00 | 15.00 ± 0.05 |
| CS-E (GlcA-GalNAc 4,6S)2 | 1.46 ± 0.05 | 1.46 ± 0.00 | 1.48 ± 0.01 |
| CS-B (GlcA 2S-GalNAc 4S)2 | 0.61 ± 0.00 | 0.62 ± 0.01 | 0.59 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark (Galeus melastomus). Mar. Drugs 2018, 16, 344. https://doi.org/10.3390/md16100344
Vázquez JA, Fraguas J, Novoa-Carvallal R, Reis RL, Antelo LT, Pérez-Martín RI, Valcarcel J. Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark (Galeus melastomus). Marine Drugs. 2018; 16(10):344. https://doi.org/10.3390/md16100344
Chicago/Turabian StyleVázquez, José Antonio, Javier Fraguas, Ramón Novoa-Carvallal, Rui L. Reis, Luis T. Antelo, Ricardo I. Pérez-Martín, and Jesus Valcarcel. 2018. "Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark (Galeus melastomus)" Marine Drugs 16, no. 10: 344. https://doi.org/10.3390/md16100344
APA StyleVázquez, J. A., Fraguas, J., Novoa-Carvallal, R., Reis, R. L., Antelo, L. T., Pérez-Martín, R. I., & Valcarcel, J. (2018). Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Catshark (Galeus melastomus). Marine Drugs, 16(10), 344. https://doi.org/10.3390/md16100344








